7 The Heart and Echocardiography
7.1 Learning Objectives
After reviewing this chapter, you should be able to do the following:
- Become familiar with some of the cardiac anatomical and physiological features.
- Understand conditions related to the heart.
- Examine ultrasound images from echocardiography.
7.2 Introduction
At various times in history, the heart has dominated much attention as the center of the function of living. Although no longer considered the center of emotions and thought, the heart continues its dominance in sonography in terms of both the frequency with which studies are done and the breadth of modalities used. Echocardiography is a rapidly growing and frequently used assessment tool in acute care settings.
7.3 Cardiac Anatomy and Physiology
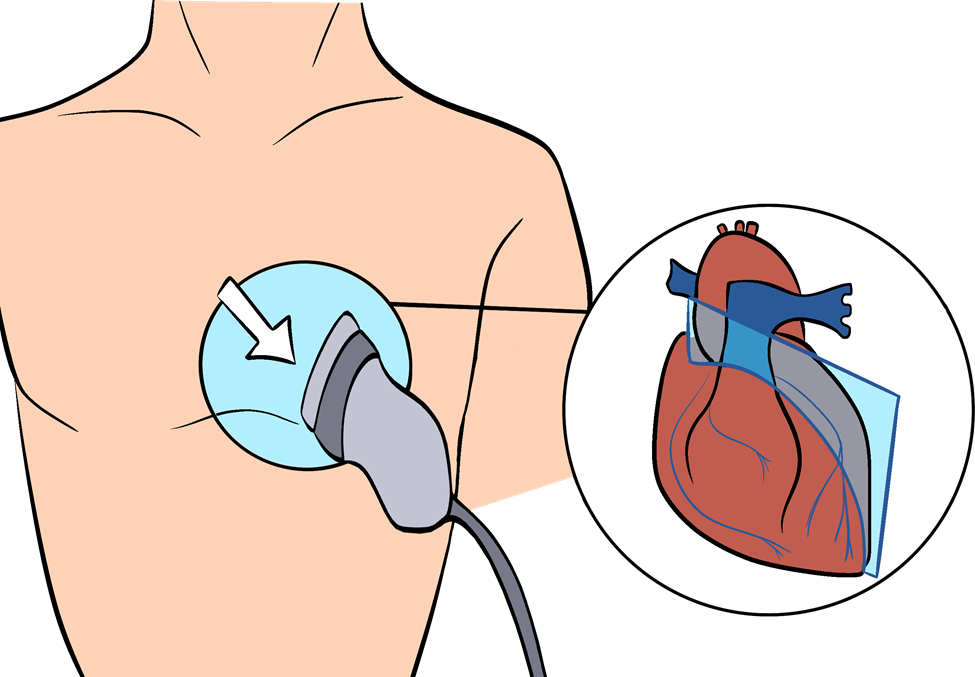
The position of the heart in the thorax is much more variable than often expected. The heart may be in a more superior or lateral placement in the thoracic cavity due to abdominal anatomical abnormalities. Lung conditions, such as emphysema, may present a heart in a vertical orientation due to chronic lung overdistension. In most healthy individuals, the heart may be best seen in a view called the parasternal long axis, where the probe is held at the second or third intercostal space immediately left of the sternum, as shown in Figure 7-1. Directly below the palpated area will be the chamber walls of the left atrium and the left ventricle.
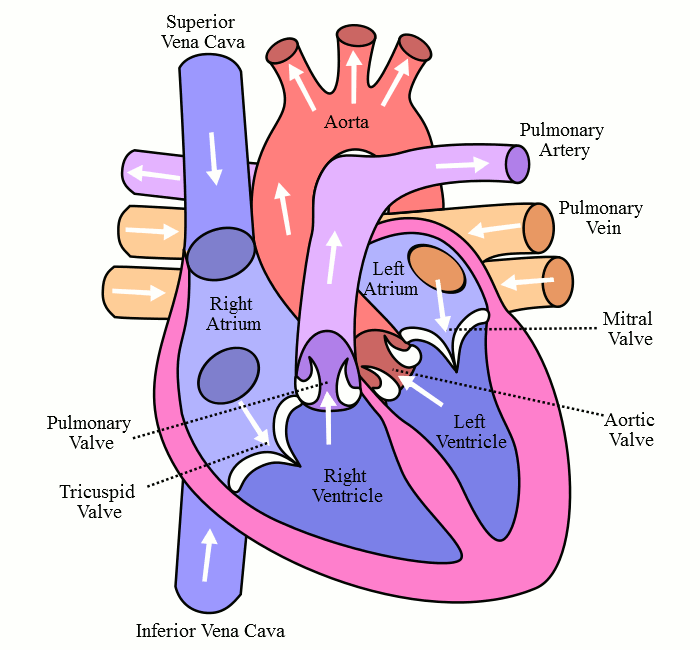
With optimal health, the heart contracts and expands synchronously. The circulation of the blood in the heart is shown in Figure 7-2. Blood enters the right atrium from the inferior vena cava and the superior vena cava. Blood then flows through the tricuspid valve to the right ventricle during cardiac expansion or diastole; this is mostly a passive flow. Blood is then expelled from the right ventricle during systole (or cardiac contraction) through the pulmonary artery and into the progressively smaller pulmonary arteries, pulmonary arterioles, and finally, pulmonary capillaries. Carbon dioxide and oxygen exchange occurs between the pulmonary capillaries and alveolar sacs of the lungs. Freshly oxygenated blood then returns via the pulmonary veins to the left atrium. Blood flows mostly passively from the left atrium, through the mitral valve, and into the left ventricle during diastole. Blood is finally propelled during systole through the aortic valve into the aorta and the body. The heart adapts to different behavioral and physiological stressors.
7.4 Echocardiography
A commonly performed, more sophisticated ultrasound evaluation of the heart is called echocardiography, often performed by technicians and cardiologists. Echocardiography machines are generally highly sophisticated (and expensive), with the capability and clarity of the views obtained improving over the years.
Some of the most sophisticated machines that are routinely used in echocardiography can evaluate wall motion of specific areas of the heart and perform static measurements of both the opening and closing of the valves that are cycling more frequently than once. Color flow Doppler features in these machines can simultaneously differentiate blood flows going through insufficient valves. These findings help determine what procedures are best for patients with heart disease. Some patient conditions and the ultrasound principles behind the evaluations are described below.
7.4.1 Ischemia
Ischemia is inadequate blood flow through the coronary arteries that may lead to myocardial (cardiac muscle cell) dysfunction or myocardial death. Ischemia typically results in chest pain. Temporary chest pain from reversible ischemia is called angina. Permanent myocardial tissue death from ischemia is called myocardial infarction. It is a common task of primary care providers to determine if the chest pain symptom an individual is experiencing is related to ischemia or another medical cause. Shortness of breath (dyspnea) is also a common symptom of ischemia. The goal is to address angina or reversible ischemia before irreversible global myocardial tissue death. The next step in addressing suspected coronary artery ischemia for patients fortunate enough to access the best health care is coronary artery catheterization. Further diagnosis and treatment with coronary artery catheterization are often done during the same procedure.[1] A coronary artery blockage can be treated with a small open mesh tube called a stent. If indicated, a stent or other procedure to reduce the blockage is completed to restore adequate blood flow.
It is common in the United States to try to diagnose those patients with risk factors and typical chest pain with an evaluation called a stress echocardiogram, also known as a “stress echo” or cardiopulmonary exercise testing with echocardiographic examination. In this test, the patient’s heart rate is increased either by having them exercise on a treadmill or bike or by pharmacological measures to increase heart rate and heart contractility.[2] Pharmacological measures are used for patients with comorbidities, such as orthopedic or neurological conditions that may prevent safe treadmill or bike use. If there is a blockage of a single vessel in a stress echo, the part of the heart that this particular vessel supplies blood to will contract less efficiently. For example, the left main coronary artery (LCA) supplies blood to the anterior part of the heart. In patients with a blockage of their LCA, the anterior portion of the heart will have noticeably less contractility. The reduction of contractility may be coupled with symptoms such as angina or dyspnea during physical movement. The contractility dysfunction that is induced by a rapid heart rate is often followed by cardiac catheterization. A coronary artery bypass grafting during an open-heart procedure may be needed in more severe cases. If so, a large incision is made in the chest to expose the heart. A vessel is removed from the patient’s leg, chest, or arm and grafted around the coronary artery with blockage.
7.4.2 Myocardial Tissue Dysfunction
Myocardial muscle function has been assessed for over 200 years, and knowledge of congestive heart failure continues to change. Without imaging, it was hypothesized that heart contractility could be impaired and that the acceptance of incoming blood would be likewise impaired. This was realized to result in increased vascular pressure and fluid leakage in the peripheral interstitium, leading to ankle edema and edema in the lungs.[3] A stethoscope was used to hear the lung edema and probably first noted in an autopsy. Treatment at first involved trying to reduce intravascular volume by using a tourniquet or bloodletting. This was done with some success. One of the first medicines to treat it was a purification of products of the foxglove plant to produce a derivative of digitalis. To this day, medical providers continue to try to decrease intravascular volume and increase cardiac contractility. Digitalis derivatives like digoxin continue to be prescribed for abnormal heart rhythms.
Echocardiography is most important in the initial diagnosis and ongoing care of reduced myocardial contractility. Heart muscle contractility is assessed for quality and calculated as an ejection fraction (EF).[4] This is calculated by measuring the area of the left ventricle during diastole when the left ventricle is at the endpoint of receiving all of the blood prior to the contraction and at the point where the left ventricle has reached end-systole, which is the greatest point of contraction. The blood volumes corresponding to the endpoints during diastole and systole are referred to as end-diastolic volume (EDVol) and end-systolic volume (ESVol), respectively. Stroke volume (SVol) is calculated by subtracting ESVol from EDVol. EF can then be mathematically expressed as follows:[5]
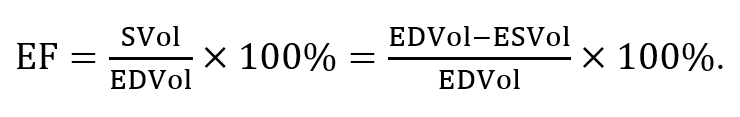
If the left ventricle contains 100 ml of blood at the end of diastole and 40 ml when it fully contracts during systole, SVol is 60 ml, and thus, the EF is 60%. A normal EF percent is considered to be around 55–75%. EF percent measurements are becoming more reliable. This is largely due to software improvements allowing the area of a 3D object to be measured on a 2D screen. The description of a patient having an EF percent of significantly less than 50% gives experienced clinicians a good idea of a patient’s morbidity and mortality.
7.4.3 Valvular Dysfunction
Heart valves allow blood to travel from one area of the heart to another and prevent “backflow” from its new area to the old area. The four valves evaluated by echocardiography are the aortic valve (between the left ventricle and aorta), the mitral valve (between the left atrium and the left ventricle), the tricuspid valve (between the right atrium and right ventricle), and the pulmonary valve (between the right ventricle and the pulmonary artery). Clinically relevant dysfunction is much more common in the aortic and mitral valves. There are two common types of valvular dysfunction.
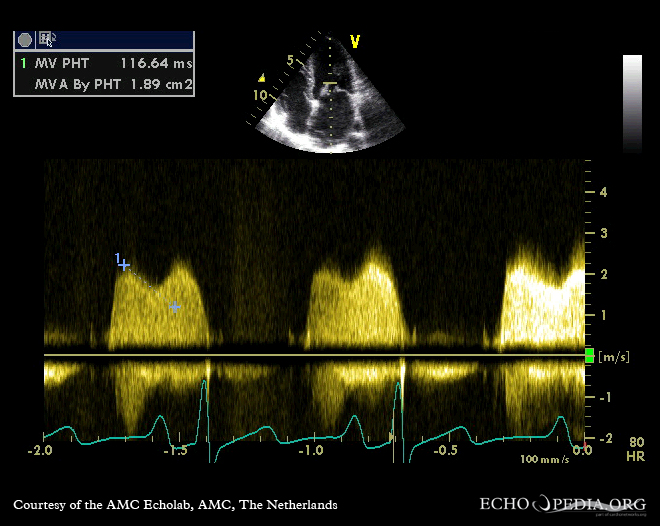
- Narrowing of the valve (stenosis) allows an inadequate amount of flow through the aortic stenosis, which may cause both inadequate perfusion and backflow pressure to the left ventricle, resulting in abnormal left ventricular enlargement. Figure 7-3 shows a severe mitral valve stenosis on echocardiography.
- Incompetence of the valve or valvular regurgitation allows the abnormal backflow of blood, causing a lack of perfusion and abnormal pressure into the contributing portion of the heart. Aortic regurgitation can be observed on echocardiograms, as shown in Figure 7-4.
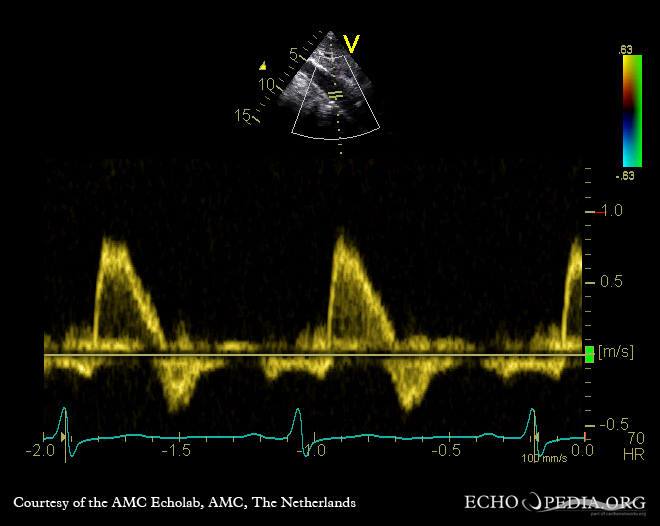
Echocardiography evaluates valvular dysfunction by direct visualizations (measuring valve diameter in systole and diastole). This is obtaining static views for those changing most often more frequently than once per second in real time. Indirect measurements are obtained by measuring blood velocity through the valve and watching the color Doppler backflow of blood through valves with regurgitation.
7.5 Self-Assessment
- What ultrasonic view gives the best access to the heart anatomy during cardiac ultrasound scanning for most healthy individuals?
- What is ischemia? How does this lead to myocardial infarction?
- How is the ejection fraction calculated?
- What are the two common types of valvular dysfunction?
7.6 Further Readings
- Singh S, Goyal A. The origin of echocardiography: A tribute to Inge Edler. Tex Heart Inst J. 2007;34(4):431–8. PMID: 18172524; PMCID: PMC2170493.
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002 Jun 19;39(12):1890–900. doi: 10.1016/s0735-1097(02)01886-7. PMID: 12084585.
- Kossaify A, Bassil E, Kossaify M. Stress Echocardiography: Concept and Criteria, Structure and Steps, Obstacles and Outcomes, Focused Update and Review. Cardiol Res. 2020 Apr;11(2):89–96. doi: 10.14740/cr851. Epub 2020 Mar 10. PMID: 32256915; PMCID: PMC7092766.
- Chhabra L, Zain MA, Siddiqui WJ. Coronary Stents. [Updated 2023 Aug 7]. In: StatPearls [internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507804/ ↵
- Chhabra L, Zain MA, Siddiqui WJ. Coronary Stents. [Updated 2023 Aug 7]. In: StatPearls [internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507804/ ↵
- Malik A, Brito D, Vaqar S, et al. Congestive Heart Failure. [Updated 2022 Nov 7]. In: StatPearls [internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430873/ ↵
- Malik A, Brito D, Vaqar S, et al. Congestive Heart Failure. [Updated 2022 Nov 7]. In: StatPearls [internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430873/ ↵
- Kosaraju A, Goyal A, Grigorova Y, Makaryus AN. Left Ventricular Ejection Fraction. 2023 Apr 24. In: StatPearls [internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 29083812. ↵

