8 Pulmonary Ultrasound
8.1 Learning Objectives
After reviewing this chapter, you should be able to do the following:
- Identify the challenges associated with pulmonary ultrasound.
- Learn the most efficient and effective techniques used in pulmonary ultrasound.
- Understand the expanded use of lung ultrasound in primary care, including COVID-19.
8.2 Introduction
As late as 1991, the most authoritative textbooks in internal medicine stated that pulmonary ultrasound was impossible due to the poor transmitting quality of air. Daniel Lichtenstein was the first to realize that all of the artifacts produced in the lungs represented different conditions, such as pulmonary edema, pneumothorax, pneumonia, and pulmonary effusion.[1] In recent years, various handheld ultrasounds have been extensively used for relatively quicker evaluations of lung conditions, including COVID-19, an infectious disease caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus that was discovered in 2019.[2]
8.3 Cardiac Versus Pulmonary Ultrasound
While both systems are housed in the thorax, pulmonary and cardiac ultrasound approaches and findings can be strikingly different. As briefly discussed in the previous chapter, cardiac ultrasound has specific measures used for valves, blood velocity, and cardiac muscle contractility. The operators’ abilities can adversely affect measurement accuracy on such subtle issues as the angle at which the probe is held and, subsequently, the angle at which measurements are obtained. One of the productive uses of artificial intelligence in the ultrasound field allows the operator to know when the best angle of insonation is obtained before the image is captured. Clear imaging is partially achieved by filtering raw images and removing portions of the images that are confusing to obtain the measurements. These measurements then determine important treatment routes, including choosing medication for the patient and even deciding if cardiac surgery is necessary.
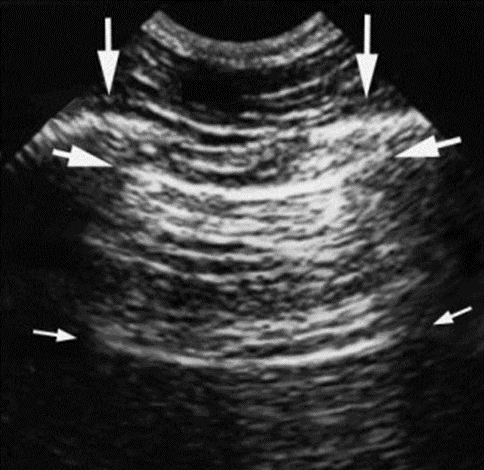
In many ways, pulmonary ultrasound is the antithesis of cardiac ultrasound. The motion cycle of the lungs is less dramatic and less frequent, and the “snow patterns” on the screen caused by a lung consolidation or other conditions allow health care providers to make rapid, critical, and often lifesaving decisions about patients who are most ill. The total picture of the reflections of an ultrasound beam that is received from the same beam-emitting probe is often blurred. The “artifacts” are often distracting and even prohibitive in making the exact measurements. Figure 8-1 shows an ultrasound image of the intercostal space. Daniel Lichtenstein, known by many as the father of critical care ultrasound, was one of the first experts to recognize that the artifacts emitted in the lungs were not the problem but part of the solution in determining the pulmonary and vascular status of the patient.[3] The notion of lung ultrasound was possible and pertinent through Dr. Lichtenstein and his colleagues’ recognition and hard work.
8.4 A Brief Pathophysiology of the Lungs
Successful respiratory cycles are controlled at many levels, from the brain to the alveoli. Clinically, we focus on what we can change to improve our patients’ conditions. The brain’s complex mechanisms and primary circuits, such as the autonomic nervous system and vagus nerve, are often out of our reach to meaningfully control for the long-term. We can mimic respiratory control by completely paralyzing our muscles and forcing air with oxygen concentrations into the lungs at a rate determined to be optimal by the clinician. This action is almost always temporary, such as for patients needing general anesthesia for surgery or patients in respiratory failure needing intensive care unit (ICU) attention. Lung ultrasound is most often used to help diagnose the cause of acute respiratory failure or evaluate continuing critical care efforts on patients already being actively treated for respiratory failure.
8.5 Ultrasound Assessment of Some Lung Conditions
Low-frequency curvilinear transducers with frequencies in the range of 3–6 MHz are best suited for lung ultrasound. Much of the recorded action of the lung begins and ends with the pleura. The pleura, a general term for the parietal pleura, air, and visceral pleura, is the denser outer covering of the lung that moves with the lung. From a sonographer’s perspective, the pleura is the most easily visualized lung structure. Inspecting the pleura sonographically allows for observations of both pleural motion and pleural sonographic disruption.
8.5.1 Disruptions of Pleural Motion
As the pleura moves, it moves the rest of the lung. This “sliding lung sign” accurately indicates lung motion and successful ventilation. If the pleura is not sliding, the lung, at least on the side of the thorax being inspected, is not ventilating thoroughly. Quick speculations about the causes of a lack of ventilation can be confirmed and treated to save lives. Below are some causes of a lack of ventilation that can be considered when there is no sliding lung sign:[4]
- Pneumothorax
- Adhesions of the surface of the pleura from infection or autoimmune disease
- Pleural effusions not allowing lung expansion
- Central or peripheral nervous system malfunction by not stimulating proper inspiration
- Neuromuscular failure impacting the diaphragm or intercostal muscles that influence adequate inspiration
8.5.2 Disruptions of Pleural Consistency
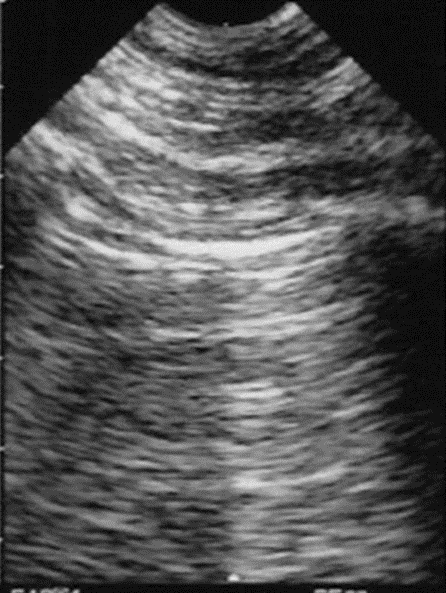
What have been named “A-lines” are the results of a reverberation artifact from the pleura or recurrent mirror images of the pleura that appear under the pleural lining at regular intervals, as shown in Figure 8-2. Disruption of the interstitium of the pleura changes the entire pleural reflection. Because the beam is prevented from uniformly penetrating the pleura, the areas that are disrupted are now seen as “B-lines,” as shown in Figure 8-3. There is no longer a reverberation artifact or A-lines when there are B-lines in motion.
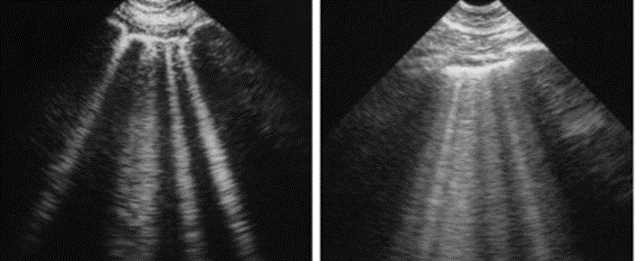
B-lines representing pleural interstitial disruption often represent pulmonary edema or vascular overload, as shown in the right diagram of Figure 8-3. The edema, which collects on the pleural surface, causes “comet tail” artifacts that account for the lines extending the lungs’ entire length. It is often in the realm of intensivists or pulmonologists to ascertain a patient’s intravascular volume. Dr. Lichtenstein was an original contributor to the direct visualization of the inferior vena cava to determine intravascular blood volume. He later discovered that B-lines might more accurately represent intravascular volume overload.
8.5.3 Lung Parenchyma
Ultrasound principles allow us to understand that the complete reflection of the waves due to necrotic debris is represented as a bright image on the screen in the area of the lung parenchyma. Nonsonographic clues such as a fever may lead the clinician to speculate that the bright reflection is an infection of the alveolar cells or pneumonia. Since alveoli have such thin walls that allow oxygen to be exchanged, it is often accurately feared that this same thin wall would easily allow infectious organisms to also cross from the alveoli to the bloodstream. Noninfectious obstructions around the alveoli appear as a similar bright reflection of the ultrasound image. This scarring is known as atelectasis and is often considered much less dangerous to the patient.
8.6 The Reciprocal Development of COVID-19 and Its Assessment
Due to many factors, it is a slippery slope to write anything about COVID-19. The disease is widespread, the actual effectiveness of isolation is unknown, testing has been inaccurate for the most part, supplies to even care for patients have been limited to an unprecedented extent in most developed countries, and the treatment theories change almost daily.
Far too many patients diagnosed with COVID-19 have had a rapid downhill course and go on to have a painfully long critical care course with a high mortality rate. For the first time in the world, there have been several considerations brought to the forefront. There has been a common consensus that COVID-19 has caused significant shortages of medical supplies both in the United States and worldwide, including those in testing capacity, ICU and hospital bed supply, hospital staff, personal protective equipment (PPE), and mechanical ventilators for affected regions.[5] There is greatly magnified attention on the safety of caretakers of the most critically ill patients. The mental health and well-being of health care professionals have been the focus of increased attention, with persistent evidence of high burnout, psychosocial stress, and mortality rates.[6] There has been a realization of how the increased vulnerability of our underserved population will perhaps impact how medical care is distributed in the future. The prevalence of COVID-19 has had a disproportionate impact on the poor, minorities, and a broad range of vulnerable populations due to its inequitable spread in areas of dense population and limited mitigation capacity resulting from a high prevalence of chronic conditions or poor access to high-quality public health and medical care.[7] There has been a growing need for more research on health equity in order to increase global knowledge and allow cross-national learning of what works for those most in need due to the direct and collateral effects of COVID-19. It has also been demonstrated that a pandemic can quickly destroy many characteristics of our society, affecting the economic, social, and personal habits of people in all countries. The World Health Organization has warned of a mental health burden related to the spread of COVID-19 infection through the global population: stress, worry, fear, and changes in our daily lives (working from home, temporary unemployment, homeschooling, etc.) are all challenging people’s mental and physical health as well as the global health care system and economy.[8]
COVID-19 has been a very humbling condition to deal with for any health care professional. Many patients do well even with a positive COVID test. Many patients found to be COVID-positive on routine screening remain asymptomatic. On the other end of the care spectrum, too many people without major risk factors are experiencing significant acute and chronic symptoms from the virus. Many have died, and a handful of them have been health care workers. The objective predictors of who will do well, such as lab tests and plain X-rays, have been inaccurate in too many cases. Especially in developed countries, diagnosticians can usually rely on numerous pieces of data to arrive at a reasonable treatment plan. At the time of this writing, COVID-19 remains a disease where the outcome can be unpredictable in a most painful manner. Patients with comorbidities are indeed at far greater risk of doing poorly, up to the point of having a much higher mortality rate. Age, congestive heart failure, chronic obstructive pulmonary disease, and dementia significantly increase the chance of doing poorly. The realization that an extensive ICU run for patients with limited life expectancy may not be in the best interest of the patient or a society where ICU resources are limited has been a most painful reality for medical workers to consider.
Lung ultrasound with handheld machines has developed into the most useful serial evaluator of the progression of COVID-19 lung disease. One of the challenges during the COVID-19 pandemic is was that several patients had to undergo ultrasound examinations within a limited amount of time, and those ultrasound tests needed to be completed on easily transportable machines. It was imperative for these machines to have the least number of knobs and small unreachable parts or spaces so that they can be sterilized quickly for immediate use with other COVID-19 patients.
Returning to the earlier chapters, we recall that degrees of brightness represent specific tissue characteristics, such as density. Pleura is one of those highly reflective densities. As the acute phase of COVID-19 disease progresses, distinctive changes may be observed first on lung ultrasound, which warns the clinician that the patient may be quickly worsening. Initially, COVID-19 may begin to change the pleura and cause the patient’s condition to worsen due to pleural thickening and focal fluid collection in some areas. Pleural thickening can be relatively easily seen on lung ultrasound, as shown in Figure 8-4.
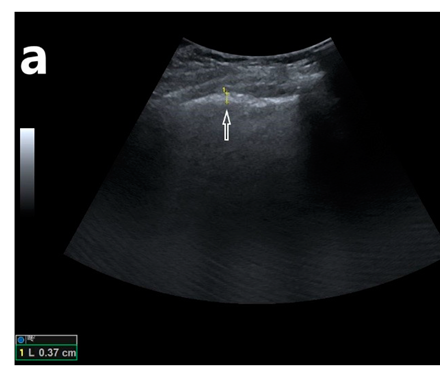
As the thickened pleura from COVID-19 becomes even more inflamed, adhesions can develop in specific areas of the lungs. These adhesions may also be focal so that there may be focal areas of impaired ventilation even on the same side of the lung. As COVID-19 disease progresses, there can be focal infiltrates in the posterior and inferior portions of the lung, which are unique and can be identified on an ultrasound as gray areas (these regions appear anechoic on the normal lungs) commonly referred to as “ground glass”. The nature of the infiltrates with “ground glass” is present. The “ground glass” is from inflammation of the bronchioles and alveoli. This type of inflammation is characteristic of COVID-19.
It has been suggested that the ultrasound or CT findings of infiltrates in certain distributions can also be used as a diagnostic test suggesting the need for imploring further specific COVID-19-influenced testing. CT almost always presents a more precise view of the anatomic effects of the COVID virus on a patient. Handheld ultrasound is more accurate in evaluating the physiological effects of COVID-19 on ventilation, especially when considering the sliding lung sign. CT is more expensive and delivers more radiation. It may not be as practical to clean thoroughly to prevent droplet transmission of infection.
As opposed to other serious systemic diseases, progressive lung findings may remain focal or become more diffuse in the lungs. As the disease progresses, a more diffuse systemic condition from adhesions and inflammation may develop. This is known as acute respiratory distress syndrome (ARDS), which often leads to the need for ventilator support for several weeks. Several characteristics of COVID-19 have forced ethical discussions of patient care to very different levels from those for patients with other conditions.
Given the dismal results of cardiopulmonary resuscitation (CPR) in treating patients with COVID and the genuine risk to presumably healthy medical professionals, modifications to CPR have been suggested by many institutions that influence health care actions. These include the following:[9]
- Do not begin CPR if there is inadequate PPE for the CPR caretakers or a lack of training in the use of PPE.
- The number of caretakers in a room where a patient with COVID-19 is undergoing CPR should be limited to active caretakers only. This is to be done even at the expense of education for the less experienced.
- CPR should be modified to not manipulate the respiratory system unless there is specialized equipment to limit COVID-19 spread. Consider compression-only CPR if bag-mask ventilation is difficult. Consider a mechanical compression device if there is a need for prolonged CPR.
- Open discussions should be had with patients who would have a low chance of out-of-hospital survival if there were cardiopulmonary arrest or other emergent conditions. It is a solemn consideration even to begin CPR on a patient with COVID-19 when there is cardiopulmonary arrest, and the chances of meaningful survival of the patient are low.
Some of the humbling parts of COVID-19 can be seen in the personal experiences of clinicians who have authentic discussions with patients with COVID-19 and the statistics of their success or failure in helping the most ill:
- Hasan et al.[10] systematically reviewed mortality in COVID-19 patients with ARDS. According to their work published in September 2020, they found that the overall pooled mortality estimate among 10,815 ARDS cases in COVID-19 patients was 39%.
- In a study conducted by Bielski et al.[11] in November 2021, there was no significant difference between pre-COVID-19 and COVID-19 periods in bystander-witnessed arrests, bystander CPR, and the use of mechanical chest compression. However, since the COVID-19 pandemic began, cardiac arrest was observed to be more frequent at home, and bystanders used automated external defibrillators less frequently.
- A 2010 review of 79 studies[12] involving 142,740 patients found that the survival rate of out-of-hospital cardiac arrest was only 7.6%. Bystander-initiated CPR may increase those odds to 10%. Survival after CPR for in-hospital cardiac arrest was slightly better[13] but still only about 17%.
- Although experience with COVID-19 continues to grow, reported mortality rates range from 50% to 97% in those requiring mechanical ventilation.[14],[15] These are significantly higher than the published mortality rates ranging from 35% to 46% for patients intubated with H1N1 influenza pneumonia and other causes of ARDS.[16],[17] Mortality was significantly associated with older age, lower body mass index, chronic renal disease, and receipt of mechanical ventilation, vasopressors, renal replacement therapy, or vasodilator therapy, among several others.
8.7 Long COVID and Ultrasound
As the pandemic progresses, chronic health outcomes associated with acute COVID are becoming more frequent and are classified under the “long COVID” category. While the acute phase of COVID-19, which is often mild or moderate, usually lasts one to two weeks, the symptoms of long COVID can last for several months and often appear to be nonspecific and not restricted to organ systems. Clarifying the underlying causes and developing a meaningful, effective, and focused diagnostic algorithm are critical. Lung ultrasound will almost certainly be an essential consideration in the care of these patients, and it has been shown to be useful for the diagnosis of COVID-19 in the acute stage of the disease.[18] Clofent et al. were able to show, in their study of 352 adult long-COVID patients with a long-term follow-up of 2–5 months, that lung ultrasound could be implemented as a first-line diagnostic procedure in the treatment course.[19] It was shown that the outcome of lung ultrasound in adults could produce good discrimination between patients with persistent abnormalities compared with high-resolution CT. However, in this population, Clofent et al. found a high rate of interstitial lung disease after acute COVID-19 infection. On the other hand, according to a study conducted on children and adolescents by Gräger et al.,[20] lung ultrasound is not suitable as a standard in the follow-up of long-COVID patients without initial pneumonia or pathological findings in an existing baseline examination. They concluded that better standard examination protocols need to be established for this patient group. Nevertheless, lung ultrasound is an important diagnostic tool for the lung because of its radiation-free nature, rapid availability, and increasing establishment in practice (with increasing experience of the examiners). In summary, much is yet to be discovered from a more adequate evaluation and treatment of worsening patients with COVID-19.
8.8 Self-Assessment
- What is referred to as a “sliding lung sign”?
- What do “A-lines” and “B-lines” represent?
- Why have handheld ultrasound units become popular in primary care in recent years?
8.9 Further Readings
- Lichtenstein DA. Lung ultrasound in the critically ill. An Intensive Care. 2014 Jan 9;4(1):1. doi: 10.1186/2110-5820-4-1. PMID: 24401163; PMCID: PMC3895677.
- Lichtenstein DA. Current Misconceptions in Lung Ultrasound: A Short Guide for Experts. Chest. 2019 Jul;156(1):21–25. doi: 10.1016/j.chest.2019.02.332. Epub 2019 Mar 11. PMID: 30872018.
- Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997 Nov;156(5):1640–6. doi: 10.1164/ajrccm.156.5.96-07096. PMID: 9372688.
- Duggan NM, Shokoohi H, Liteplo AS, Huang C, Goldsmith AJ. Best Practice Recommendations for Point-of-Care Lung Ultrasound in Patients with Suspected COVID-19. J Emerg Med. 2020 Oct;59(4):515–520. doi: 10.1016/j.jemermed.2020.06.033. Epub 2020 Jun 12. PMID: 32713618; PMCID: PMC7290164.
- Cascella M, Rajnik M, Aleem A, et al. Features, Evaluation, and Treatment of Coronavirus (COVID-19) [Updated 2023 Aug 18]. In: StatPearls [internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/
- Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997 Nov;156(5):1640–6. doi: 10.1164/ajrccm.156.5.96-07096. PMID: 9372688. ↵
- Cascella M, Rajnik M, Aleem A, et al. Features, Evaluation, and Treatment of Coronavirus (COVID-19) [Updated 2023 Aug 18]. In: StatPearls [internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/ ↵
- Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997 Nov;156(5):1640–6. doi: 10.1164/ajrccm.156.5.96-07096. PMID: 9372688. ↵
- Lichtenstein DA. Lung ultrasound in the critically ill. An Intensive Care. 2014 Jan 9;4(1):1. doi: 10.1186/2110-5820-4-1. PMID: 24401163; PMCID: PMC3895677. ↵
- Dar M, Swamy L, Gavin D, Theodore A. Mechanical-Ventilation Supply and Options for the COVID-19 Pandemic. Leveraging All Available Resources for a Limited Resource in a Crisis. Ann Am Thorac Soc. 2021 Mar;18(3):408–416. doi: 10.1513/AnnalsATS.202004-317CME. PMID: 33202144; PMCID: PMC7919160. ↵
- Gupta N, Dhamija S, Patil J, Chaudhari B. Impact of COVID-19 pandemic on healthcare workers. Ind Psychiatry J. 2021 Oct;30(Suppl 1):S282–S284. doi: 10.4103/0972-6748.328830. Epub 2021 Oct 22. PMID: 34908710; PMCID: PMC8611576. ↵
- Shadmi E, Chen Y, Dourado I, Faran-Perach I, Furler J, Hangoma P, Hanvoravongchai P, Obando C, Petrosyan V, Rao KD, Ruano AL, Shi L, de Souza LE, Spitzer-Shohat S, Sturgiss E, Suphanchaimat R, Uribe MV, Willems S. Health equity and COVID-19: Global perspectives. Int J Equity Health. 2020 Jun 26;19(1):104. doi: 10.1186/s12939-020-01218-z. PMID: 32586388; PMCID: PMC7316580. ↵
- World Health Organization (WHO). Mental Health and COVID-19: Early evidence of the pandemic’s impact. [place unknown]: Mental Health and Substance Use, WHO Headquarters; 2022 Mar 2. 13 p. Report No.: WHO/2019-nCoV/Sci_Brief/Mental_health/2022.1. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1 ↵
- Nolan JP, Monsieurs KG, Bossaert L, Böttiger BW, Greif R, Lott C, Madar J, Olasveengen TM, Roehr CC, Semeraro F, Soar J, Van de Voorde P, Zideman DA, Perkins GD; European Resuscitation Council COVID-Guideline Writing Groups. European Resuscitation Council COVID-19 guidelines executive summary. Resuscitation. 2020 Aug;153:45–55. doi: 10.1016/j.resuscitation.2020.06.001. Epub 2020 Jun 7. PMID: 32525022; PMCID: PMC7276132. ↵
- Hasan SS, Capstick T, Ahmed R, Kow CS, Mazhar F, Merchant HA, Zaidi STR. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: A systematic review and meta-analysis. Expert Rev Respir Med. 2020 Nov;14(11):1149–1163. doi: 10.1080/17476348.2020.1804365. Epub 2020 Sep 29. PMID: 32734777; PMCID: PMC7544968. ↵
- Bielski K, Szarpak A, Jaguszewski MJ, Kopiec T, Smereka J, Gasecka A, Wolak P, Nowak-Starz G, Chmielewski J, Rafique Z, Peacock FW, Szarpak L. The Influence of COVID-19 on Out-Hospital Cardiac Arrest Survival Outcomes: An Updated Systematic Review and Meta-Analysis. J Clin Med. 2021 Nov 27;10(23):5573. doi: 10.3390/jcm10235573. PMID: 34884289; PMCID: PMC8658174. ↵
- Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010 Jan;3(1):63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. Epub 2009 Nov 10. PMID: 20123673. ↵
- Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS; American Heart Association Get with the Guidelines–Resuscitation Investigators. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012 Nov 15;367(20):1912–20. doi: 10.1056/NEJMoa1109148. PMID: 23150959; PMCID: PMC3517894. ↵
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium; Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020 May 26;323(20):2052–2059. doi: 10.1001/jama.2020.6775. Erratum in: JAMA. 2020 May 26;323(20):2098. PMID: 32320003; PMCID: PMC7177629. ↵
- Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020 Apr 28;323(16):1612–1614. doi: 10.1001/jama.2020.4326. PMID: 32191259; PMCID: PMC7082763. ↵
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016 Feb 23;315(8):788–800. doi: 10.1001/jama.2016.0291. Erratum in: JAMA. 2016 Jul 19;316(3):350. Erratum in: JAMA. 2016 Jul 19;316(3):350. PMID: 26903337. ↵
- Estenssoro E, Ríos FG, Apezteguía C, Reina R, Neira J, Ceraso DH, Orlandi C, Valentini R, Tiribelli N, Brizuela M, Balasini C, Mare S, Domeniconi G, Ilutovich S, Gómez A, Giuliani J, Barrios C, Valdez P; Registry of the Argentinian Society of Intensive Care SATI. Pandemic 2009 influenza A in Argentina: A study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. 2010 Jul 1;182(1):41–8. doi: 10.1164/201001-0037OC. Epub 2010 Mar 4. PMID: 20203241. ↵
- Oks M, Cleven KL, Cardenas-Garcia J, Schaub JA, Koenig S, Cohen RI, Mayo PH, Narasimhan M. The effect of point-of-care ultrasonography on imaging studies in the medical ICU: A comparative study. Chest. 2014 Dec;146(6):1574–1577. doi: 10.1378/chest.14-0728. PMID: 25144593. ↵
- Clofent D, Polverino E, Felipe A, Granados G, Arjona-Peris M, Andreu J, Sánchez-Martínez AL, Varona D, Cabanzo L, Escudero JM, Álvarez A, Loor K, Muñoz X, Culebras M. Lung Ultrasound as a First-Line Test in the Evaluation of Post-COVID-19 Pulmonary Sequelae. Front Med (Lausanne). 2022 Jan 13;8:815732. doi: 10.3389/fmed.2021.815732. PMID: 35096906; PMCID: PMC8794580. ↵
- Gräger S, Pfirschke R, Lorenz M, Vilser D, Krämer M, Mentzel HJ, Glutig K. Lung ultrasound in children and adolescents with long-term effects of COVID-19: Initial results. Front Pediatr. 2023 Mar 24;11:1112881. doi: 10.3389/fped.2023.1112881. PMID: 37033176; PMCID: PMC10080098. ↵

