14 Respiratory System
Elska Kaczmarek; Jackson Phillips; and Bill Ryerson
Focus Questions—to Guide Your Reading of This Chapter
- What are the main differences between water and air as respiratory media? How are those differences reflected in the respiratory structures of aquatic and terrestrial animals?
- Although water and air differ in some key ways, what fundamental anatomical features of respiratory structures are important for effective diffusive gas exchange regardless of respiratory medium?
- Ways of breathing, or the active ventilation of respiratory organs, are also incredibly diverse across vertebrates. Why do you think that breathing behaviors vary so much, and why does the movement of water or air across a respiratory surface matter for gas exchange?
14.1 Introduction
One of the easiest ways to recognize the importance of your respiratory system is to hold your breath. At first, nothing feels wrong. However, you will quickly feel a tightening of your chest, and your body will begin to fight you. Your chest will start to rise and fall; even as you hold your mouth closed, you will feel a strong need to breathe, until your body finally ignores your mind and breathes in and out several times quickly. In rare cases, people can even lose consciousness at this point, which is why we do not recommend trying it at home. Why do we need to breathe so badly and so often?
Our respiratory systems fulfill a fundamental physiological need: gas exchange. At the cellular level, mitochondria perform cellular respiration, converting sugars and other fuels into ATP (raw energy), and this process uses up oxygen (O2) and produces carbon dioxide (CO2). As this happens, our cells require fresh oxygen deliveries and a way to remove the carbon dioxide in order to maintain homeostasis. This is true for all animals. The main way that gases move in and out of cells is diffusion. Diffusion is a relatively simple process, but it is incredibly important to how animal bodies function. Matter that is in a fluid state is fluid because individual particles are freely moving past each other. This movement is what creates diffusion, such that when different concentrations of particles meet (e.g., a drop of red dye in a cup of undyed water), the concentrations even out over time, simply due to the random movement of particles (Figure 14.1). This is the basic principle behind gas exchange: Expose low-oxygen blood to a high-oxygen source, and oxygen will diffuse into the blood (and CO2 will diffuse out).
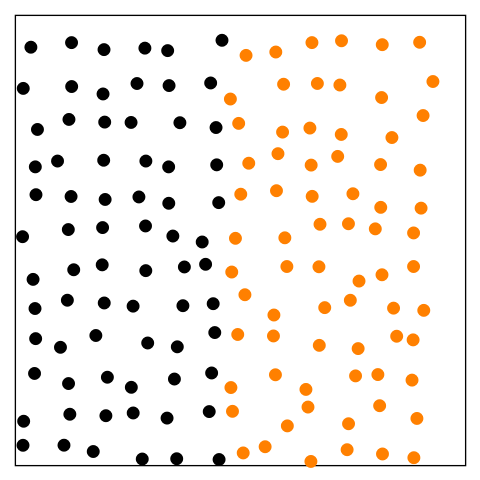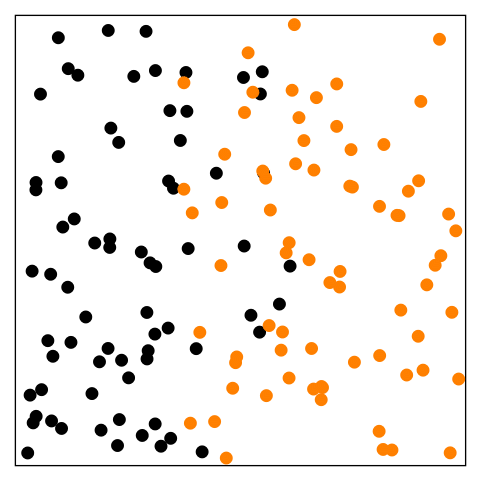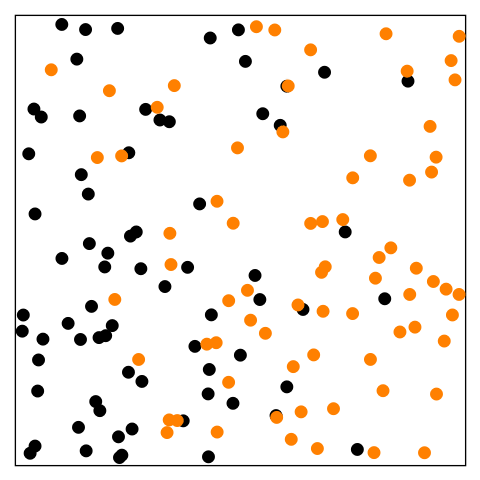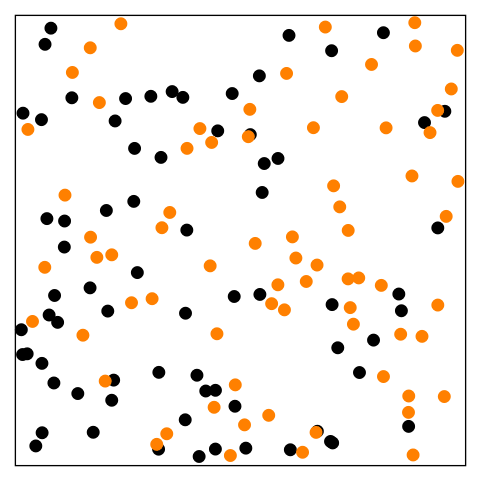
Figure 14.1—An animation illustrating the basic principle of diffusion. At the beginning, the black dots are on the left and the orange dots are on the right. The dots begin to move randomly and are interspersed by the end.
For a simple, very small animal (e.g., a tardigrade), diffusion from the outside environment alone might be sufficient for gas exchange. Indeed, many small invertebrate animals have no specialized respiratory organs, and oxygen simply diffuses in through their skin. However, this strategy gets harder and harder as animals get bigger. This is a scaling issue, caused by the geometric relationships between surface area and volume (Figure 14.2, but also see Chapter 5). As 3D objects get bigger, volume grows faster than surface area, meaning that larger bodies have lower relative surface areas, making diffusion less efficient. Another problem is that for oxygen to pass through a surface, like skin, that surface must be thin and moist. This is potentially a problem for animals that need defensive structures like scales or want to avoid water loss (the dry skin of desert-living frogs). The way that most animals, including nearly all vertebrates, deal with these problems is to have specialized respiratory organs with large surface areas that do the vast majority of the heavy lifting of gas exchange. The classic respiratory organs seen in vertebrates are gills and lungs, but skin is also an important site of gas exchange for many animals (see Section 14.2, General Structure and Function).
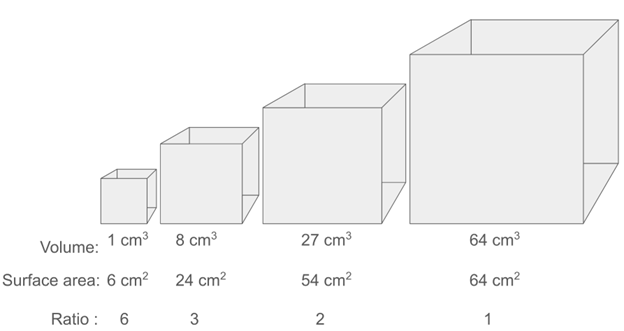
Figure 14.2—The scaling relationship between surface area and volume, illustrated with a series of cubes of increasing size. Note that the surface-area-to-volume ratio decreases as the cube gets larger.
Such specialized respiratory organs also rely on a vascular system to transport the absorbed oxygen to the brain and muscles and transport carbon dioxide back to the respiratory organ, to be released into the environment. In vertebrates, this is, of course, powered by the heart, which maintains blood flow through the body. The heart partitions freshly oxygenated blood, which is pumped to the body, and low-oxygen blood, which is pumped to the respiratory organ. This partitioning is a neat trick—by only sending low-oxygen blood to the respiratory membranes, the blood is oxygenated more quickly because of the large difference in concentrations between the blood and the environment. The greater that difference, the faster the change.
Another way that animals increase the efficiency of gas exchange is by controlling and coordinating the flow of environmental oxygen over respiratory organs. Basically, by breathing! There is a reason we do not just hold our mouths open and passively diffuse oxygen down our throats and into our lungs. By moving air into and out of respiratory organs, we can maintain concentration gradients by replacing spent air or water. In the cases of countercurrent and crosscurrent exchange, we can even use flow at the site of diffusion to speed up diffusion even further.
The way that animals exchange oxygen and carbon dioxide with their environment is dependent on many factors, but perhaps the most fundamental is the respiratory medium: water versus air. Earth’s atmosphere is roughly 20.95% oxygen, 78% nitrogen, 1% argon, and just .04% carbon dioxide! That is a lot of oxygen, all produced by Earth’s amazing photosynthesizers (plants and algae), and it is available 24/7, 365 days a year. This abundance of atmospheric oxygen is what allows us humans, and other terrestrial animals, to exist. In the water, the dynamics are quite different. Oxygen must be dissolved in water to be accessed by an aquatic animal. Unlike in the atmosphere, oxygen does not make up a significant volume of a lake or stream. Instead, oxygen levels vary throughout the day and part of the year as well as between types of water bodies. And even a bottle of water that has been shaken vigorously to fully saturate it with oxygen has hundreds of times less oxygen than the same volume of air. This means that for many aquatic animals, the primary struggle of gas exchange is consistently getting enough oxygen. For terrestrial animals, oxygen is constantly available, and the more pressing concern is often getting rid of carbon dioxide. In fact, the reason that holding your breath is painful is that your CO2 levels are too high, not that there is not enough O2. Luckily for fish and other aquatic animals, carbon dioxide is highly soluble in water and so readily diffuses out of their gills.
Many different forms of respiratory organs have evolved across vertebrates. The most common respiratory organs today, lungs and gills, are also some of the oldest, having evolved in the ancestors of modern fish hundreds of millions of years ago. Other respiratory strategies are more recent, having evolved in specialized groups. The common themes across the respiratory system are (1) differences in water-based versus air-based respiration; (2) increases in surface area of the respiratory surface; and (3) mechanisms to increase concentration gradients during gas exchange, such as ventilation and coordinated blood flow patterns. Take a breath and dive into the fascinating world of vertebrate respiration.
14.2 General Structure and Function
There are a diverse array of respiratory structures and ventilation behaviors across vertebrates. Later in this chapter we will explore this diversity, but in this section, we will give an overview of the most common respiratory structures and focus on shared properties across vertebrates.
Types of Respiratory Organs
Vertebrate respiratory organs can be grouped into three major categories: gills, air-breathing organs, and respiratory skin (Figure 14.3). Each category faces its own set of physical constraints and demands that influence its morphology and mode of ventilation.
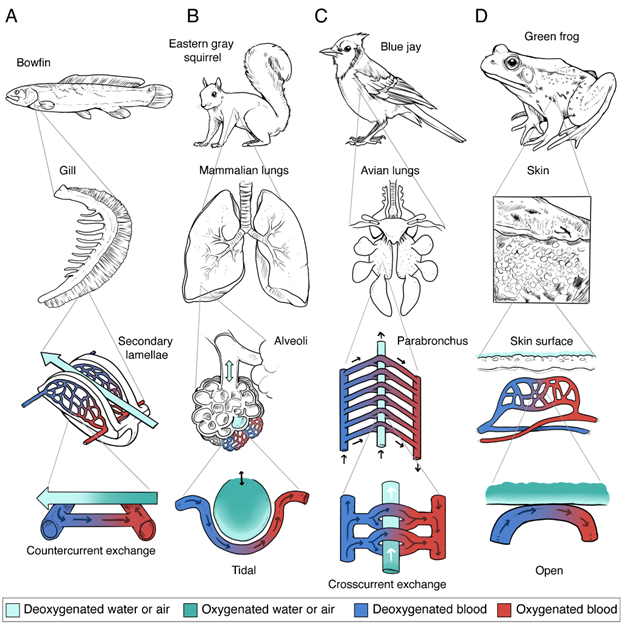
Figure 14.3—Illustrations of the basic anatomy of four examples of respiratory structures: fish gills (A), mammalian lungs (B), avian lungs (C), and amphibian skin (D). These are shown at different scales: representative organism (top row), gross anatomy of the respiratory structure (second row), microanatomy of the respiratory structure (third row), and the site of diffusion between the capillaries and the respiratory media (fourth row). Arrows indicate the direction of movement of the blood or respiratory media (water or air). Light teal—deoxygenated respiratory fluid. Dark teal—oxygenated respiratory fluid. Navy blue—deoxygenated blood. Red—oxygenated blood.
Gills
With few exceptions, gills are used for gas exchange in water. They are therefore equipped to deal with the high density and comparatively low oxygen availability of water. Water is much denser and more viscous than air, and so it requires more work to move (i.e., ventilate) water than air. However, ventilation maintains concentration gradients and promotes diffusion and so is quite important. To make matters worse, water contains a fraction of the oxygen contained in air. For example, at 20°C and at sea level, air contains 210 mL of O2 per L of air, whereas freshwater contains just 6.6 mL of O2 per L of water. The amount of oxygen dissolved in water depends on the partial pressure of oxygen (pO2) in the air above the water and on the solubility of oxygen in water, which is fairly low and dependent on temperature and salinity. At high altitude, air pressure decreases, so pO2 decreases accordingly, and in warmer and saltier water, the solubility of oxygen decreases, creating a lot of variation in how much oxygen is in different water bodies.
The high density of water causes gill ventilation to be energetically expensive. Therefore, gill ventilation must be efficient to offset this energetic cost. In a similar vein, the limited amount of oxygen in water means that gill-breathers often need to maximize their diffusive capacity in order to support their metabolic needs. Gills achieve this by (1) having a large surface area, (2) being ventilated with a unidirectional flow of water, and (3) in many groups, having capillaries arranged to facilitate countercurrent exchange.
Gills have a large surface area because they are covered in small, vascularized folds. The specific design of these folds can vary—for example, the gills of larval actinopterygian fish have thin, filamentous processes, whereas the gills of adult fish have flat plates—but, because these folds are vascularized, they all contribute to a large respiratory surface area. This large surface area is important both because it increases the surface area for diffusion and because it causes flow rate to slow down, increasing the time during which diffusion can take place. Specifically, this is because the velocity of a fluid decreases as it moves from a narrow to a wider region. The combined cross-sectional area of the branchial arteries is smaller than that of the vascular channels within the gills. Similarly, the cross-sectional area of the pharynx is smaller than the combined cross-sectional area of the passages between the folds of the gills. These mismatches cause the velocities of both their blood and the water in the gills to slow down, increasing time for diffusion of gases.
Unidirectional flow of water over the gills supports an efficient exchange of respiratory gases because it provides a continuous supply of fresh, oxygenated water to the vascular surface where exchange occurs. If gills were more like human lungs and were ventilated bidirectionally—that is, tidally—then half of each ventilation cycle would be spent flushing out water that can no longer be used as a source of oxygen—not an efficient use of energy. Unidirectional flow also enables countercurrent exchange, as we will discuss next.
The relative paths of ventilated water and circulated blood in the gills are also critical to their function. For a moment, let us consider what would happen if blood (within the gills) and water (flowing across the gills) were flowing in the same direction. Let us assume that the water starts well oxygenated (10 mg/L), the blood starts out totally anoxic (0 mg/L), and there are equal amounts of water and blood. As the two streams flowed alongside one another, oxygen would diffuse from the water into the bloodstream, and the amount of oxygen in the blood and water would eventually reach equilibrium at some intermediate value (~ 5 mg/L). Unfortunately, in this case, half of oxygen remains in the water! To avoid this, gills use a pattern of flow known as countercurrent exchange (Figure 14.3), which is a clever trick for maximizing diffusion and exchange. During countercurrent exchange, blood flows in the opposite direction of water. Therefore, at every point of contact along the path of the blood and the water, the water has a higher concentration of oxygen (and lower concentration of carbon dioxide) than the blood that it is passing, and so the gases keep diffusing the whole time. For example, as a parcel of water loses oxygen to adjacent blood flowing through gill capillaries, the slightly deoxygenated water flows toward blood that has just entered the gills and contains even less oxygen than the water, enabling further diffusion. In this way, the blood and water never reach equilibrium, and diffusion continues until very little oxygen remains in the water.
Because gills create close contact between capillaries and the surrounding water, heat, water, valuable salts, and nitrogenous wastes also readily diffuse across respiratory gill membranes. In freshwater fishes, water diffuses into the body (toward the higher salt concentration), and in marine fishes, water diffuses out (again, toward the higher salt concentration). As a result, most fishes cannot maintain a body temperature that is warmer than the water they are swimming in, and they require physiological adaptations to maintain internal osmotic balance.
Air-Breathing Organs
Breathing air is, in many ways, the exact opposite of breathing water. Air is oxygen rich, has low density, and has low viscosity. Carbon dioxide is highly soluble in water and readily diffuses from the gills but is not very soluble in air. Like gills, water readily diffuses across the respiratory membrane of air-breathing organs. While not problematic for aquatic air breathers, water loss across lung membranes during air breathing poses a major challenge for terrestrial air-breathing vertebrates.
The low density of air means that respiratory membranes, and any elaborations that increase its surface area, must be supported to prevent collapse. This is the reason gills are not effective in air, because their filaments rely on water to support them, and on land they clump together. On the other hand, the low density of air makes it much easier to move (ventilate), and its high oxygen content means that gas exchange doesn’t need to be incredibly efficient. These two properties make bidirectional tidal ventilation and low ventilation rates possible. Air can easily be moved in and out of an air-breathing organ, unlike water, which has high inertia and viscosity. Additionally, the high oxygen concentration gradient between blood and atmospheric air means that there is plenty of diffusive capacity for most animals’ metabolic demands without needing countercurrent exchange. In some taxa, including birds, more complete gas exchange is necessary and is facilitated by adaptations such as unidirectional airflow and crosscurrent exchange. Crosscurrent exchange is similar in some ways to countercurrent exchange. In this case, however, the flow of blood is perpendicular to the flow of ventilated air, maintaining concentration gradients more effectively than in typical tidal flow. We see this system in bird lungs (Figure 14.3).
The earliest known vertebrate air-breathing organs evolved in bony fish, allowing these ancestral fishes to store a pocket of air inside their bodies and then continue swimming about. Extant fishes and tetrapods possess lungs and respiratory gas bladders that have evolved from these ancestral air-filled organs. Like lungs, respiratory gas bladders are air-filled sacs that are vascularized and conduct gas exchange with the atmospheric air that is breathed into them. Unlike tetrapod lungs, gas bladders are unpaired, composing a single air bladder. The internal location of lungs and respiratory gas bladders is useful for air-breathing fishes because they can store air for gas exchange. In amphibious and terrestrial vertebrates, this internal location is advantageous because the respiratory membrane remains moist while water loss is reduced. Air within lungs contains a lot of water vapor (evaporating from the moist respiratory surface), but water loss is reduced by the low rate of ventilation and low proportion of lung volume that is exchanged with each breath. In addition, air is “conditioned” in the airways before and after entering the lungs, reducing how much moisture leaves the body.
A diverse array of air-breathing organs has evolved in fishes much more recently than the origin of lungs. These organs are subject to the same constraints described above. They must allow the fish to store a pocket of air, the respiratory membranes must be supported, and low ventilation rates are sufficient, given the high proportion of oxygen in atmospheric air compared to water.
Skin
Aquatic, amphibious, and terrestrial species have evolved adaptations that allow skin to be used as a respiratory organ, most often (but not always) as a supplement to the gills or lungs. In aquatic species, respiratory skin faces similar constraints as gills: The low oxygen availability in water makes it especially important for the respiratory membrane to have a large surface area and maintain a high concentration gradient by vascularizing the surface with low-oxygen blood. In terrestrial species, respiratory skin could just as well be classified as an air-breathing organ and faces the challenges faced by the lungs of terrestrial vertebrates: The respiratory membrane must remain moist but reduce water loss, and CO2 is not very soluble in air. In addition, skin serves numerous other functions (see Chapter 6), many of which preclude the use of the skin for respiration. Adaptations that enable skin to act as a respiratory organ include having capillary beds positioned close to the surface of the skin and having accessory skin structures (such as vascularized folds or other projections) that increase surface area (Figure 14.4).
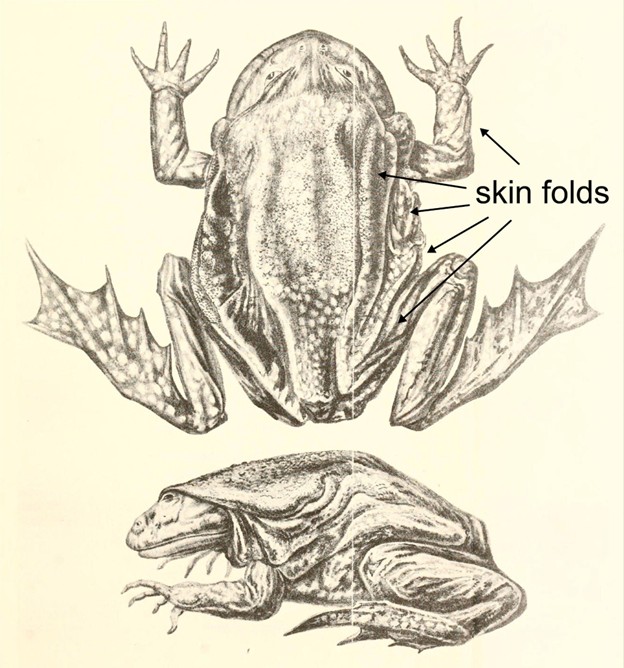
Figure 14.4—An illustration of the Lake Titicaca frog Telmatobius culeus. Arrows point to the excess skin and skin folds on the surface of the frog.
Mechanisms of Ventilation
The mechanisms for ventilating respiratory structures can be grouped into three major categories: oropharyngeal pumping, aspiration pumping, and nonpumping body movement. Although we have presented three categories of respiratory structures (gills, lungs, and skin) and now three categories of ventilation mechanisms, they do not match up one-to-one. Oropharyngeal pumping is used to ventilate gills, but it is also used to ventilate air-breathing organs in fish and amphibians. Similarly, body movement can be used to ventilate the surface of the skin, but it is also used for ram ventilation to ventilate the gills of some fish. Aspiration pumping is exclusively a lung-breathing mechanism found within tetrapod vertebrates.
Oropharyngeal Pump
Oropharyngeal pumping uses a combination of expansion and compression of the oral and/or pharyngeal (and neighboring) cavities to produce pressure, driving the flow of fluid (water or air) into and out of the mouth. Variants of this general mechanism are mostly used by fishes and amphibians to ventilate their gills, lungs, gas bladders, and other air-breathing organs. Different forms of oropharyngeal pumping mechanisms have different names based on exactly which cavities are used for ventilation. A dual pump is used to ventilate the gills of fishes, and it involves two cavities: the buccal cavity and the parabranchial (in sharks) or opercular (in bony fishes) cavity. Air breathing in fishes and amphibians uses expansion and compression of just a single cavity, the buccal cavity, and so is called a buccal pump.
The dual pump produces unidirectional flow over the gills (Figure 14.5A). Described simply, the buccal cavity expands and draws water into the mouth through the open jaws, while the parabranchial/opercular cavity (the name of this cavity differs in sharks and bony fish, respectively) expands and draws water past the gills. Then the buccal cavity compresses and pushes water past the gills, and the parabranchial/opercular cavity compresses and pushes water out of the gill cavities. This cycle repeats to create pulses of unidirectional flow over the gills.
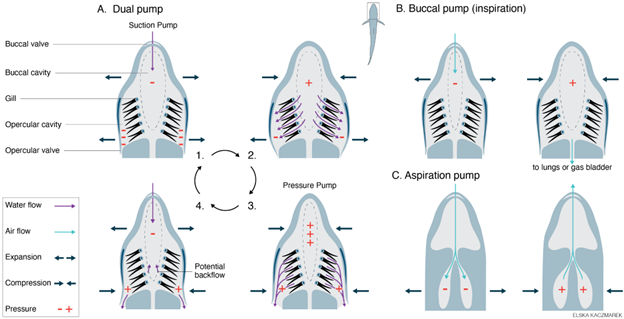
Figure 14.5—Ventilation mechanisms used for water breathing and air breathing: dual pump (A), buccal pump (B), and aspiration pump (C). (A) The dual pump generates unidirectional flow of water over the gills via the cyclical expansion and compression of the buccal cavity and opercular cavity (or parabranchial cavity in sharks). Note that pressure is always lower in the opercular cavity than the buccal cavity. (B) The buccal pump is used to inspire air into the lungs or respiratory gas bladder via the expansion and compression of the buccal cavity. (C) The aspiration is used to inspire and expire air into the lungs of amniotes via expansion and compression of the thorax and/or abdomen. Purple arrows—water flow. Teal arrows—airflow. Dark blue arrows—movement of the animal’s head or body. Orange plus and minus symbols—water or air pressure relative to the ambient pressure.
During air breathing, the buccal pump must move air from the mouth into the air-filled organ (lungs or gas bladder) rather than out via the gill openings, as in the dual pump (Figure 14.5B). This is accomplished by closing the gill openings (i.e., opercular valves) and by opening the glottis to allow air into the lungs or gas bladder. During inspiration, the buccal cavity expands and draws air into the mouth through the open jaws, and then the mouth is compressed after the jaws shut, pushing air into the lungs or gas bladder through the open glottis. Thus, buccal pumping produces inspiration, one-half of the tidal flow that occurs during air breathing.
Expiration of gas (the second half of tidal flow) is not caused by buccal pumping. In fishes, expiration is caused by hydrostatic pressure on the body wall, elastic recoil of the lung/gas bladder walls, and possibly the contraction of smooth muscle in the lung walls. In contrast, in some amphibians, expiration is caused by contraction of a hypaxial muscle (transverse abdominis) to compress the body wall and lungs. This is called an “expiration pump.” When an amphibian is submerged in water, hydrostatic pressure on the body wall also contributes to expiration. (Note that although we have discussed the mechanism of inspiration first and then expiration, in fish and amphibians, expiration of gas typically occurs first, followed by inspiration of new air.)
In air-breathing fishes that do not use lungs or gas bladders (and instead use other diverse air-breathing organs), a buccal pump is typically used to drive airflow, but the pattern of movement may differ from what is described above.
Aspiration Pump
Unlike oropharyngeal pumping, aspiration uses active contraction of muscles in the thorax and abdomen to change lung volume and produce tidal ventilation (Figure 14.5C). During aspiration, the pressure driving airflow is generated by muscles that surround the lungs—the lungs are inside the pump, not on one end of it. Rather than air being forced into the lungs using compression of the mouth, as occurs during buccal pumping, air is sucked or aspirated into the lungs using expansion of the thorax. Muscular compression of the thorax and elastic recoil of the lung walls cause exhalation. The muscles used for aspiration include intercostal muscles, hypaxial muscles, and/or a muscular diaphragm, depending on the taxa.
This may sound familiar because, as we briefly discussed above, contraction of the hypaxial muscles is the mechanism of expiration in some amphibians. The use of axial muscles for expiration (expiration pump) is hypothesized to be an evolutionary intermediate between the use of cranial muscles for inspiration (buccal pump) and the use of axial muscles for both exhalation and inhalation (aspiration pump). The evolution of the aspiration pump frees the head from the functional demands of producing inspiration, allowing morphological and functional diversification of cranial structures (see Section 14.6, Integration).
Nonpumping Body Movement
This category serves as a catchall for alternative forms of movement-based ventilation aside from oropharyngeal pumping and aspiration. For example, amphibians with external gills contract muscles at the bases of the gills to wave the gills back and forth through the water. The Lake Titicaca frog (Telmatobius culeus) has loose folds of skin that it uses for cutaneous respiration in its high-altitude home, and it will perform jerky push-up-like movements to create water flow over its skin and increase gas exchange (Figure 14.4). Many pelagic fish species and some sharks hold their mouths slightly open while swimming, allowing water to flow passively over the gills. This is called ram ventilation, and although the drag created by the open mouth reduces swimming efficiency, it saves the energy that would be spent using the dual pump for gill ventilation.
14.3 Development of the Respiratory System
Being equipped to perform respiratory gas exchange is crucial through all stages of life. Not only must newly hatched or newborn animals have at least one functional respiratory organ to survive, but developing embryos must also have an effective mechanism for gas exchange. In this section we will discuss how gills and lungs arise during embryonic development, how embryos themselves perform gas exchange, and how lungs are stretched throughout development so that they will be healthy and ready for a lifetime of breaths.
Development of Gas-Exchange Organs
Vertebrate gills arise from a mix of ectoderm and endoderm on the pharyngeal arches. They typically appear during the embryonic stage, becoming fully functional soon after hatching into the water. Animals with aquatic eggs, like fishes and amphibians, typically have an aquatic larval stage and greatly depend on cutaneous gas exchange and their gills to survive to adulthood. In vertebrates with a biphasic life cycle (where the organism metamorphoses between the larval and adult stages), like frogs and salamanders, the gills are usually resorbed at metamorphosis in anticipation of the move onto solid land.
During embryological development, lungs and gas bladders form as endodermal outpocketings from the foregut (Figure 14.6). Lungs are typically (but not always) paired and bud off the ventral side of the foregut, whereas the gas bladder is unpaired and buds off the dorsal side of the foregut. The connective tissues of the respiratory system are derived from mesodermal tissue groups, forming the vasculature, smooth muscle, and cartilaginous elements of the lungs. The hollow connection to the mouth or foregut is called the trachea (in tetrapods) or pneumatic duct (in nontetrapod fishes). The opening to both the trachea and pneumatic duct is called the glottis, which is closed and opened via muscular control.
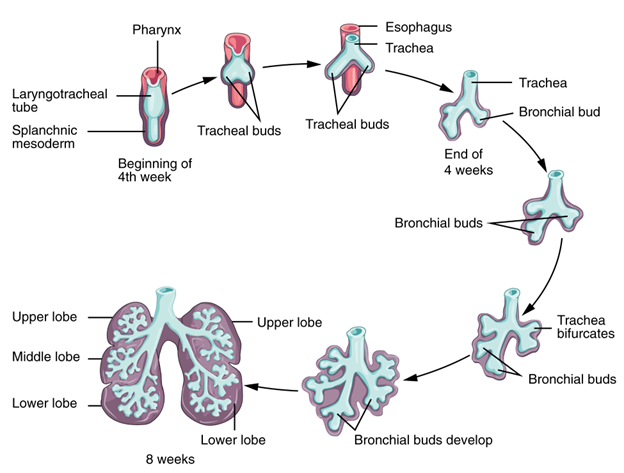
Figure 14.6—The branching development of the lungs in humans and other mammals. Human lungs develop as small buds on the ventral side of the anterior gut tube, and then successively branch, like a tree, over the next four weeks.
Embryonic Gas Exchange
Animals have gas-exchange requirements for their entire life cycle, not just when they are adults. This creates a tricky situation: How is an animal supposed to acquire oxygen while it is still building (i.e., developing) its lungs or gills?
For many fish and amphibians, the struggle for gas exchange begins when aquatic eggs are fertilized and laid in the water (Figure 14.7). For these tiny developing embryos, oxygen must diffuse through any jelly coatings to reach the developing embryo, where oxygen diffuses either directly through the skin or via gill-like structures (Figure 14.8). Because diffusion is most effective when there is a large surface-area-to-volume ratio, and because water is relatively oxygen poor, oxygen diffusion is a major limiting factor for these groups. Often embryos must hatch at small sizes and early developmental stages to avoid asphyxiation. Some amphibians have special adaptations for gas exchange in the egg, such as endosymbiotic algae that live in the egg and are passed down across generations. These algae perform photosynthesis, using up carbon dioxide and nitrogenous wastes excreted by the developing embryo, and provide oxygen in return (Figure 14.7). In many embryonic fish and amphibians, long gill-like structures branch out from the embryo to the surface of the egg to facilitate gas exchange. In others, adults stick around after mating to fan the eggs, creating flow, which maintains concentration gradients of oxygen, allowing for diffusion to take place.

Figure 14.7—Salamander eggs from a vernal pool. The faint green color comes from symbiotic algae that photosynthesize, producing oxygen used by the developing embryos. In other groups, oxygen must diffuse through the jelly coatings of the egg mass, limiting egg size.
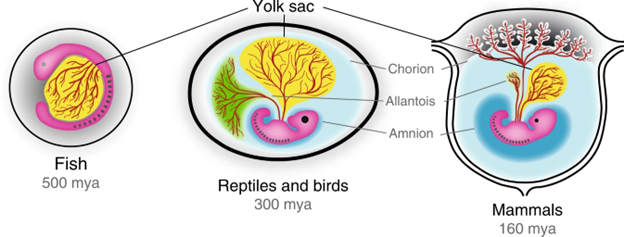
Figure 14.8—The diversity of egg types in vertebrates. Amphibian eggs are similar to the “Fish” egg shown here, while reptiles, birds, and mammals all have the amniotic condition. In nonmonotreme mammals, the external egg has been lost. The dates shown indicate estimates of when this egg type first evolved.
Animals with hard-shelled, terrestrial eggs, such as nonavian reptiles, birds, and monotreme mammals, take a different approach to embryonic gas exchange. These groups are all part of the Amniota, a group defined by their egg type (Figure 14.8). Like all animals, developing amniote embryos also have gas-exchange requirements, but unlike many fish or amphibian eggs, amniote eggs are terrestrial, meaning they are exposed to the atmosphere. On one hand, this is advantageous for gas exchange, as there is far more oxygen available in the atmosphere than is dissolved in water bodies. On the other hand, however, amniote eggs must resist desiccation while still allowing for oxygen diffusion, a fundamental trade-off in respiratory biology you have seen throughout this chapter. Amniote eggs use a semipermeable shell to deal with this trade-off, which allows gases to diffuse while keeping water inside the egg. Amniote embryos also have two extraembryonic membranes (the allantois and chorion) that facilitate gas exchange between the embryo and the external atmosphere, leading many to call amniotic eggs “air-breathing.” The evolutionary origin of the amniotic egg was an important step that permitted this lineage to spend more of their life cycle away from water and to diversify and radiate in new ways.
In humans and other placental mammals, the chorion and allantois fuse to form the placenta, which facilitates gas exchange from the bloodstream of the mother to the developing fetus. Oxygen is taken in by the mother’s lungs and then transported to the placenta, where it diffuses into the fetal bloodstream.
Box 14.1—Intersections Between Respiration, Development, and Conservation Biology
For any living creature to persist in any habitat for very long, their basic physiological needs must be met. For vertebrate animals, this means they need food to sustain themselves, enough water to keep them from drying out and to facilitate waste removal, and finally, oxygen to breathe. For aquatic animals especially, this last requirement can be a big problem. As we have covered throughout this chapter, hypoxia (low levels of oxygen) is a common occurrence for many aquatic habitats and fishes and amphibians that depend on dissolved oxygen. Aquatic adult animals deal with hypoxia in myriad ways, from coming to the surface to breathe air to using complex countercurrent exchange systems in the gills that extract every bit of oxygen dissolved in the water. However, developing fish and amphibian eggs, embryos, and larvae sometimes do not have this luxury. Often, these developmental stages strongly depend on naturally high environmental levels of oxygen. Here, we will discuss what happens when we humans change the environment, impacting the natural oxygen levels of streams and the animals that call them home.
Along the Pacific coast of the United States, Coho salmon were once an iconic feature of the landscape and the lives of Indigenous peoples of the region. Today, Coho salmon are extirpated across much of their native range, especially in California. While many factors have contributed to this decline, one major contributing factor that remains a “hot-button” issue today is related to respiratory biology during development. Salmon spend much of their adult lives out in the open ocean but return to inland freshwater streams to breed. Breeding adults swim from the ocean upstream to their breeding grounds, where they spawn millions of eggs that develop into fry and then juveniles before eventually returning to the ocean. As salmon develop, they are highly susceptible to low oxygen levels. When water levels in their breeding streams fall, so too do oxygen levels, sometimes leading to mass mortality and failed breeding events. This is particularly damaging for Coho salmon, which are semelparous, meaning they only reproduce once in their lives.
There are several ways that humans impact water levels in streams across the world. Groundwater feeds streams at their sources, and irrigation in California commonly uses groundwater from underground “aquifers.” Agriculture is incredibly important to the economy of modern California, but there is a heavy toll that using all that water takes on the natural ecology of the landscape. Aquifers are replenished by rain, but increasingly common and severe droughts predicted by models of climate change have also contributed to lower groundwater levels. During an especially severe drought from 2011 to 2016, salmon populations were decimated across California, largely because developing salmon did not survive the low-oxygen conditions found in their streams.
Understanding the respiratory physiology of organisms, especially during development, is a critical component of understanding how to conserve the natural world. If organisms’ physiological needs cannot be met, they will not persist in nature. When humans alter the natural landscape, it is critical for us to understand how those alterations impact the living creatures around us. In the case of Coho salmon, there are now much stricter regulations in place to protect the streams that still host breeding populations, and there are even places where the salmon are coming back to their ancestral breeding grounds. By understanding the respiratory requirements and then taking action to ensure those needs are met, humans have the power to help endangered organisms persist into the future.
Lung Development
Nearly all tetrapod vertebrates depend on lungs for gas exchange as adults. Lungs are interesting from a developmental perspective because inflation is an important step in lung development. In other words, lung tissue must be stretched in order to properly develop. What does this mean? Tissue development is a very complicated process, likely beyond the scope of this course. In brief, however, tissue development is controlled by complex cascades of gene expression. This means that one gene might control another gene, which might control another gene or set of genes, and organs develop correctly when the right set of genes is turned on at the right time. In lungs, some of those genes do not turn on unless the tissue is “stretched.” Experiments have shown that living lung tissue in a petri dish will not develop correctly unless it is pulled taut.
In amphibians, most lung development happens during the larval stage, such that air breathing starts while the larval frog or salamander is still reliant on gills for gas exchange. This means that the lungs can develop, be stretched out, and become functional gas-exchange organs without needing to be the primary source of oxygen during this process. However, in some cases, lungs are also important gas-exchange organs for larval amphibians, especially in hypoxic conditions. When amphibians metamorphose, they leave the water and lose their gills, and their lungs are fully functional and ready for the terrestrial world.
Amniote vertebrates do not have the luxury of a prolonged larval stage during which to stretch out and develop their lungs. Instead, they must emerge from the egg or womb with fully developed lungs that are ready to go. It is actually a remarkable feat that human babies are able to breathe within seconds of being born, considering that their lungs need to be stretched during development. It turns out that mammal embryos actually fill their lungs with liquid while in the womb, performing “fetal breathing movements” where that liquid is breathed in and out of the lungs. This stretches the lungs, and experiments in sheep and other animals have shown that stopping these fetal movements prevents proper development. This research has been applied to human medicine in efforts to prevent lung birth defects. Other amniotes, such as Anolis lizards, are similar to humans and other mammals, in that the lungs are inflated with liquid while in the egg, where the lung fully develops in anticipation of hatching.
14.4 Evolution of the Respiratory System
The evolutionary history of the vertebrate respiratory system is one full of twists and turns (literally in some cases!). While the earliest vertebrate ancestors lacked lungs and probably had primitive gills, this story is not a stepwise journey from fishlike gills to human lungs. Remember, there are no living ancestors of vertebrates, and all living organisms have had an equally long and fascinating evolutionary history (Figures 14.9 and 14.10).
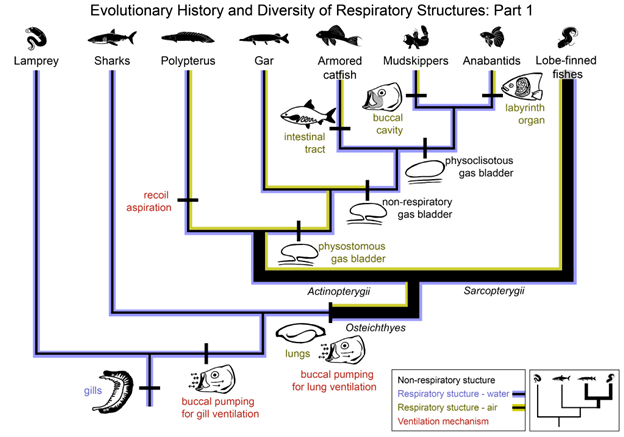
Figure 14.9—Evolutionary history and diversity of respiratory structures: Part 1. The phylogeny of living vertebrates (including Cyclostomata, Chondrichthyes, and Osteichthyes) is shown, with an emphasis on the diversity of respiratory structures within the Actinopterygii branch of Osteichthyes. Relatively few actinopterygian species can breathe air; however, we overrepresent air-breathing species here in order to highlight the diversity of air-breathing structures present in Actinopterygii. Evolutionary origins or transitions in respiratory structures (purple—structures for water breathing; yellow—structures for air breathing) or ventilatory mechanisms (red) are labeled. Branches are highlighted to indicate the respiratory medium used by each lineage (purple—breathe water; yellow—breathe air; purple and yellow—breathe water and air, “bimodal breathers”).
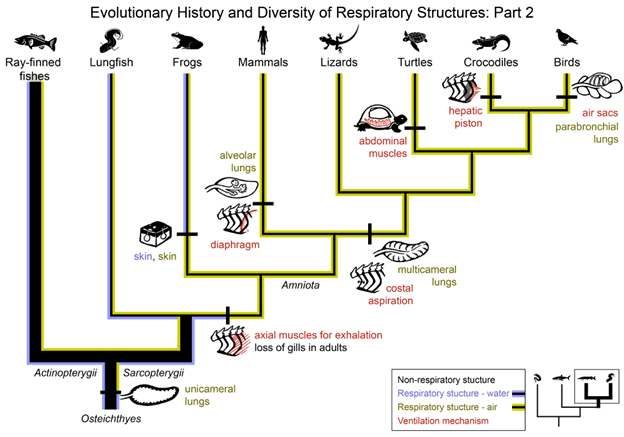
Figure 14.10—Evolutionary history and diversity of respiratory structures: Part 2. The phylogeny of Osteichthyes (Actinopterygii and Sarcopterygii) is shown, with an emphasis on the diversity of respiratory structures within the Sarcopterygii branch. Evolutionary origins or transitions in respiratory structures (purple—structures for water breathing; yellow—structures for air breathing) or ventilatory mechanisms (red) are labeled. Branches are highlighted to indicate the respiratory medium used by each lineage (purple—breathe water; yellow—breathe air; purple and yellow—breathe water and air, “bimodal breathers”).
Jawless Fishes
The earliest common ancestors of modern vertebrates swam in warm seas more than 500 million years ago. These fishlike animals lacked true teeth and had not yet even evolved jaws, though they had mouth openings. However, these animals had gill pouches to breathe water just like hagfishes and lampreys today.
The ability to breathe water through gills requires a mechanism for moving water into the mouth and over the gills. An ancient mechanism for creating water flow is the cyclic expansion and compression (in other words, pumping) of the oropharyngeal and gill chambers. Expansion draws water in through the open mouth, and compression pushes the water out past a set of vascular gills. Water flow ventilates the gills, greatly increasing the efficiency of gas exchange, as opposed to simply exposing the gills to the water around a fish. This pumping mechanism is key to understanding the transitions in ventilation strategy across all vertebrates.
Unidirectional flow of water over the gills also enabled the evolution of countercurrent flow. While water is pumped over the gills in one direction, capillaries are arranged so that blood flows in the opposite direction. This design greatly increases the rate and efficiency of gas exchange.
Gnathostomes
The next major transition in the vertebrate tree of life was the evolution of jaws, seen today in all living gnathostomes (up to and including you and me). The first jawed fishes retained the pumping mechanism used for gill ventilation in their jawless relatives but lacked a bony skeleton. Roughly 450 million years ago, these early jawed fish split into two major groups that are still alive today: the cartilaginous fish (sharks and rays) and bony fish (basically everything else).
Chondrichthyes
The cartilaginous fish for the most part retain many respiratory characteristics of early jawed vertebrates, including five gill slits (typically), no air-breathing organs, and an absence of dense, ossified bone.
Osteichthyes
The bony fishes are an extremely diverse group, including typical fishes, like the perches and trout of the world, and bizarre, terrestrial fishes that lack gills, wear shirts, and do their taxes (the Osteichthyes as a formal group includes all descendants of bony fish, and therefore includes amphibians, reptiles, and even mammals). However, their earliest common ancestors were very different from the bony fish around today.
A major development during early bony fish evolution was the origin of air breathing. Some early bony fish, which evolved to use oropharyngeal pumping (specifically the dual pump) for gill ventilation, came to use the buccal pump to breathe air (see Section 14.2, General Structure and Function, for a discussion of oropharyngeal pumping). Exactly what purpose the first air breaths served is not well understood today, though both respiration and buoyancy control have been cited as likely original functions of air breathing. This was probably a very early event in bony fish evolution, because both major lineages of bony fish alive today (ray-finned and lobe-finned fishes) have air-breathing organs (Figures 14.9 and 14.10). Among ray-finned fishes (most typical fishes), some have paired, respiratory lungs and others have unpaired, respiratory gas bladders. Like lungs, respiratory gas bladders are vascularized and conduct gas exchange with the atmospheric air that is breathed in. On the other hand, nearly all lobe-finned fishes (lungfishes and tetrapods) have paired, respiratory lungs. The fact that the earliest diverging extant lineage of ray-finned fishes also has paired lungs has led many scientists to suggest that the ancestor of all bony fishes had lungs as well and that lungs evolved into the gas bladders seen in most ray-finned fishes today.
Regardless of the directionality, it is clear that gas bladders (including nonrespiratory gas bladders known as swim bladders) and lungs are homologous, meaning they are descended from the same ancestral structure. It is possible lungs evolved first (and unpaired gas bladders evolved from them), lungs evolved multiple times from unpaired gas bladders, or lungs and gas bladders evolved independently multiple times from some third structure that served as a precursor.
There is an interesting and somewhat mysterious difference worth noting in the ventilation patterns of air breathing between ray-finned and lobe-finned fishes. Both groups ancestrally used a buccal pump, but there are major differences in the way this buccal pump is deployed (Figure 14.11). The ray-finned fishes generally use a “four-stroke” breath type: (1) the oropharynx expands to expire gas from the lungs/gas bladder, (2) compresses to push that gas back out of the mouth, (3) expands again to draw new air into the mouth, and then (4) compresses a final time to push that air into the air-filled organ. The lobe-finned fishes, on the other hand, use a “two-stroke” breath. Lobe-finned fishes like lungfish use a single expansion of the oropharynx to both expel air from the lungs and draw fresh air into the mouth. Following this, two-stroke breathers compress the oropharynx to fill the lungs back up under positive pressure. With few exceptions, lobe-finned fishes (lungfish and tetrapods) use two-stroke air breaths, and ray-finned fishes use four-stroke air breaths. This has led researchers to hypothesize that these breath types are ancestral to each lineage. It is unknown whether a four-stroke or two-stroke breath is ancestral to the other, but it has been suggested they both evolved from simpler expiration and inspiration breaths that were common to both lineages.
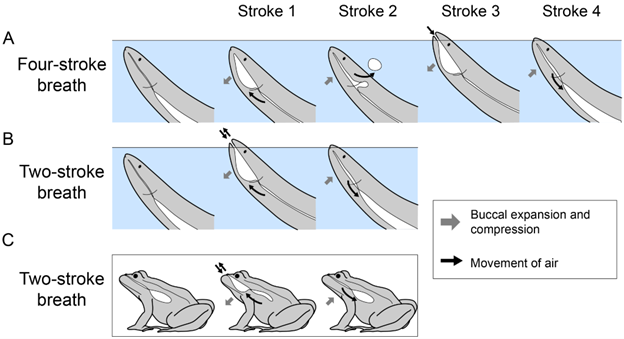
Figure 14.11—The phases of two-stroke and four-stroke air breathing. Each stroke is an expansion or compression of the buccal cavity. (A) During four-stroke breaths, strokes 1 and 2 produce expiration of air, and strokes 3 and 4 produce inspiration of air. (B) During two-stroke breaths, stroke 1 produces expiration and stroke 2 produces inspiration. Note that A and B are illustrated for a generic fishlike animal, but no single species is known to take both two-stroke and four-stroke air breaths. (C) Two-stroke breaths are also used by amphibians (using the same pattern of movement shown in B). Gray arrows—expansion or compression of the buccal cavity. Black arrows—movement of air.
Actinopterygians
While the gas bladders of only some fishes are respiratory (i.e., vascularized and used for gas exchange), all gas bladders (both respiratory and nonrespiratory) are hydrostatic because the gas inside them provides buoyancy. In fact, for most species, the gas bladder is nonrespiratory and is solely hydrostatic (i.e., functioning to increase and regulate buoyancy). Some of these species (called physostomous) retain a connection between the swim bladder and the mouth, while most species (called physoclistous) lose that connection and instead fill the bladder with nitrogen gas.
However, even among physoclistous fish, which cannot use their swim bladders for respiration, air breathing has reevolved many times. This has produced a diversity of air-breathing organs, including elaborately modified gills, respiratory membranes within the mouth, and even respiratory portions of the digestive tract (Figure 14.9). The evolution of various forms of air breathing is a common theme across the fish tree of life.
Sarcopterygians
Lobe-finned fishes, which include lungfishes and tetrapods, are nearly universally lunged. For these groups, lungs are typically the primary site of gas exchange, with some exceptions. The fascinating coelacanths (Latimeria) are an amazing group of deep-water lobe-finned fishes that were first described from fossils that are millions of years old and were considered to be a fascinating but extinct group of early sarcopterygians. Amazingly, fresh specimens of these giant deep-sea dwellers were later discovered by South African scientists in the bycatch of local fishermen in 1938, and now much more is known about this mysterious group. Coelacanths lack functional lungs and use their gills for respiration. They do, however, have small fatty organs that scientists believe to be vestigial lungs, similar to how your coccyx is a vestigial tail. The Australian lungfish, Neoceratodus, is another lobe-finned fish with a unique respiratory apparatus. Unlike other lungfishes, Neoceratodus has a single, unpaired lung and retains a lot of gill functionality. This lungfish appears to mostly use its lung as a buoyancy organ, and only when aquatic oxygen levels drop does it use its lung as a respiratory organ to supplement its gills.
Generally, however, lobe-finned fishes are quite reliant on their lungs to meet their respiratory needs. Perhaps because of this very fact, the most significant evolutionary event in the history of this group was the water-to-land transition where amphibious, tetrapod-like animals like the famous Tiktaalik began moving onto land for the very first time. This transition was only possible because these aquatic animals already had lungs and so could breathe air and acquire oxygen on land (Figure 14.10). These early land-dwellers likely shared many characteristics with modern amphibians and probably maintained an aquatic larval phase reliant on gills for respiration.
Tetrapods
While some of the early lobe-finned fishes went on to become lungfishes, retaining many “fishy” characters, others eventually evolved into the tetrapods of today: amphibians, reptiles (including birds), and mammals. These groups all are distinct in various ways with different respiratory strategies, lung morphologies, and ventilation patterns.
The amphibians evolved from early tetrapods and retain many respiratory traits that are probably similar to those groups. Most amphibians retain an aquatic larval phase, with gills of various shapes and sizes depending on the species. Amphibians also retain the ancestral buccal pump, which they use to ventilate the gills as larvae and then lungs as adults. Some amphibians, however, use muscular contractions in the body wall to actively empty the lungs in conjunction with the buccal pump used to fill the lungs. This is a major transition in ventilatory mechanics foreshadowing the transitions that would occur in other tetrapod groups. Amphibian lungs are generally simple and unicameral, consisting of two open chambers connected to a glottis at the base of the mouth. Amphibian lungs are vascular and somewhat septate, but the degree of complexity greatly differs across species.
Amphibians are the most variable tetrapod group in terms of respiratory strategy, perhaps because they employ their skin as another major source of gas exchange. Many amphibians even use their skin as the primary source of gas exchange. Others use gills as a primary source of gas exchange, not only during the larval stage, but all the way through adulthood. There are also actually amphibian species that have evolved to lose their lungs entirely, using only their skin and the lining of the mouth for gas exchange. Lacking gills, some aquatic amphibians have evolved to increase skin surface area, making gas exchange faster and alleviating some of the physical limitations of diffusion that make skin respiration difficult at large sizes (Figure 14.4). Others have evolved to retain their larval gills into adulthood, a developmental process called “neoteny.”
The final extant major tetrapod group are the amniotes, which include reptiles, mammals, and other related but now-extinct groups like the dinosaurs. The amniotes are distinguished by their key evolutionary innovation: the terrestrial, amniotic egg (see Section 14.3, Development of the Respiratory System). Early amniotes were fully terrestrial, laying eggs on land and relying on lungs for essentially all gas exchange. Modern amniotes generally retain these traits, with some key exceptions.
A major feature of amniote respiration is what is called aspiration breathing. Aspiration breathing is a set of ventilatory patterns defined by the use of active, muscular movement of the thorax and abdomen to pull air directly into the lungs. This pattern of breathing differs from the ancestral buccal pump because air moves directly into the lungs and does not require head musculature to contribute to ventilatory flow. Most modern reptiles use rib musculature to expand the body wall and pull air into the lungs. Mammals have an alternative breathing system, which uses a muscular diaphragm to expand the pleural cavity, lowering pressure in the lungs and pulling air in.
Amniote lungs vary greatly in shape. The lungs of the first amniotes were probably similar to those of amphibians: paired and unicameral. In the course of amniote evolution, other lung types have arisen, from the elongate, unpaired lung in snakes, to elaborate bird lungs with many chambers that allow for unidirectional airflow, to the branching, tree-like lungs of modern mammals. This explosion of diversity is most often explained by the higher metabolic rates of some amniotes, which require more oxygen and therefore lungs that are more complex and effective at extracting oxygen.
14.5 Diversity of the Respiratory System
The evolutionary history of the vertebrate respiratory system is a tale with many changes and innovations, shaped by changes in the ecology and physiology of ancestral species. This history has produced rich diversity in the respiratory structures and ventilation mechanisms of vertebrate species alive today (Figures 14.9, 14.10, and 14.12). In this section, we will dive in and explore this diversity in more detail.
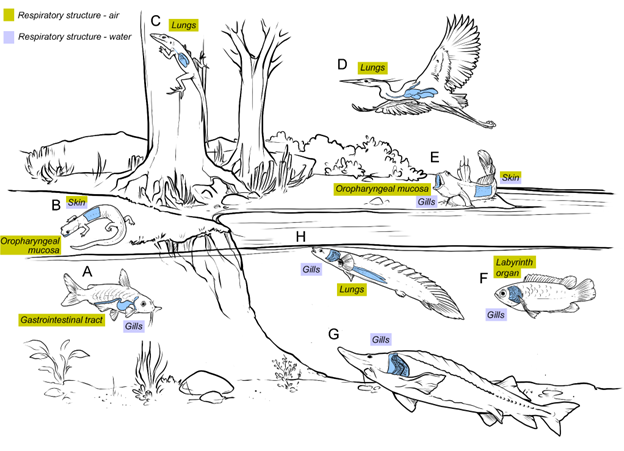
Figure 14.12—Illustration of vertebrates and their respiratory structures in a variety of habitats. Air-breathing structures are found in fully aquatic, amphibious, and fully terrestrial species, while water-breathing structures are found in aquatic and amphibious species. (A) Armored catfish—gills (water) and gastrointestinal tract (air). (B) Lungless salamander—skin (water and air) and oropharyngeal mucosa (air). (C) Basilisk lizard—lungs (air). (D) Great blue heron—lungs (air). (E) Mudskipper—skin (air and water), oropharyngeal mucosa (air), and gills (water). (F) Climbing gourami—gills (water) and labyrinth organ (air). (G) Sturgeon—gills (water). (H) Bichir—gills (water) and lungs (air).
Fishes
Gills
Gills are paired structures that have capillary-rich membranes with a large surface area and that enable respiratory gas exchange in aquatic environments. The gills of fishes (cyclostomes, elasmobranchs [sharks and rays], and actinopterygians) share many features in common but also differ in notable ways, as described in Figure 14.13. The gills and ventilation mechanism of hagfishes are the most different from the other clades, so we will discuss them last.
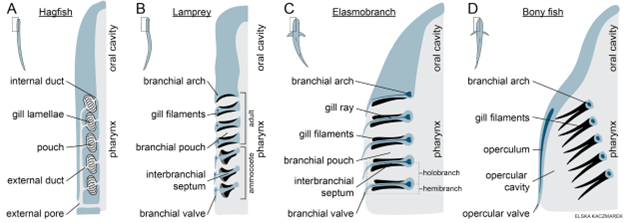
Figure 14.13—Anatomy of gills in fishes. (A) Hagfish gills are located in branchial pouches. (B) Lamprey gills are arranged in holobranchs supported by branchial arches and interbranchial septa. Larval lamprey (ammocoetes) have branchial valves to maintain unidirectional flow, while adult lamprey lack branchial valves and use tidal flow. (C) Shark gills are arranged in holobranchs supported by branchial arches and interbranchial septa. (D) Bony fish gills are supported by branchial arches, but the gill filaments extend freely without interbranchial septa.
Lamprey, elasmobranchs, and bony fishes have gills arranged as paired holobranchs (Figure 14.13). “Hemibranch” refers to one set of gill filaments, while “holobranch” refers to both sets supported by the same branchial arch (also known as a gill arch). A branchial arch is a paired series of bones that articulate dorsally on or near the head and meet ventrally along the midline. Two sets of gill filaments (primary lamellae) extend from the branchial arch, one on the anterior side and one on the posterior side. The space between the hemibranchs on adjacent branchial arches is called a branchial pouch.
In lamprey and elasmobranchs, an interbranchial septum (connective tissue) lies between and anchors the anterior and posterior sets of gill filaments of each holobranch (Figure 14.13). Elasmobranchs have a cartilaginous gill ray within each interbranchial septum, providing structural support. Elasmobranchs and larval lamprey have branchial valves (flaps that extend posteriorly and laterally from each holobranch) that create separate gill openings for water to exit through after passing through the branchial pouches. Importantly, the branchial valves also maintain unidirectional flow of water—they are pushed open when water flows out of the branchial pouches but are pushed closed by the reverse flow of water. Adult lamprey do not have branchial valves, which enables tidal ventilation, crucial for their ability to respire while feeding.
In contrast to lamprey and elasmobranchs, in bony fish, the anterior and posterior sets of gill filaments extend from the branchial arch separately, with no interbranchial septum (Figure 14.13). Instead of water flowing through distinct branchial pouches and then exiting through separate openings (divided by the branchial valves), in bony fish, water flows past each set of gill filaments and then combines and flows out of a single (typically large) opening on each side of the head: the opercular valve. The opercula are paired flat bones that cover the gills and move during ventilation. If you have ever watched a bony fish in an aquarium, you have likely seen its opercula flare out and in with every ventilation cycle. Like the branchial valves of lamprey and elasmobranchs, opercular membranes (flaps of skin extending from each operculum) function as valves that maintain unidirectional flow of water.
The gill filaments of lamprey, elasmobranchs, and bony fish are covered in tiny secondary lamellae, platelike ridges that contain respiratory capillary beds. Water flows over the secondary lamellae in the opposite direction as the blood flowing in the capillaries. This countercurrent arrangement maximizes gas exchange, and as a result, fish are able to extract a higher percentage of oxygen from their respiratory fluid than all other vertebrates. This feat is important to their survival because water contains much less oxygen than air.
Gill Ventilation
Countercurrent exchange requires unidirectional flow of ventilated water. Fishes generate unidirectional flow of water using compression and expansion of structures in the head (with the exception of adult lamprey—which use tidal, not unidirectional, flow—and species that use ram ventilation instead of cyclic movements of cranial elements).
Larval lamprey ventilate their gills using their branchial basket and their velum. The branchial basket, also known as the branchial apparatus, consists of the branchial arches and associated structures in the pharynx. The velum is a structure with muscular flap-like folds positioned between the pharynx and the mouth. To pump water out of the pharynx through the gill openings, the velum seals closed (blocking flow back into the mouth) and the branchial basket compresses. To draw water into the branchial basket through the mouth, the velum and branchial basket relax and the branchial basket elastically springs back into its expanded shape. While the velum is always used to generate flow, the branchial basket contracts and expands more when the larval lamprey has greater oxygen demand. In contrast, if the branchial basket is not contributing, then there is little flow laterally over the gills, and flow is instead used for filter feeding.
Postmetamorphosis, adult lamprey generate tidal flow (in and out of the gill openings) using only contraction and relaxation of the branchial basket. The velum separates ventilatory flow in the pharynx from the mouth. In species that are parasitic feeders as adults, this enables the lamprey to respire while its mouth is attached to the side of a fish as it feeds on its prey.
Elasmobranchs and bony fish produce unidirectional flow by compressing and expanding their buccal chamber and their parabranchial (in elasmobranchs) or opercular (in bony fishes) chambers (Figure 14.5). This mechanism is often called a dual pump because it involves two chambers or is called a “two-phase pump” because it has a suction phase and a pressure phase.
The suction phase begins with the chambers compressed and the oral and parabranchial/opercular valves closed. The buccal chamber begins to expand, the oral valve opens, and then the parabranchial/opercular chamber expands. Because the parabranchial/opercular valves remain closed, the expansion of the parabranchial/opercular chamber creates subambient pressure that is lower than that in the buccal chamber. Therefore, water flows through the buccal chamber into the parabranchial/opercular chamber, following the pressure gradient. During the pressure phase, the oral valve closes, the buccal chamber begins to compress, and then the parabranchial/opercular cavity begins to compress. Because the oral valve is closed but the parabranchial/opercular valves are pushed open, there is greater pressure in the buccal cavity, causing water to flow into the parabranchial/opercular chamber and out of the gill openings. The parabranchial/opercular valves limit reversal of flow, but flow reversals have been observed in some species, especially when there is lower respiratory drive.
Elasmobranchs that are benthic will often bury themselves in the sediment and cannot use their mouth for ventilation. Instead, they draw water in through their spiracles, which are small paired openings on the dorsal surface of the head that open into the buccal chamber. Some bottom-dwelling sharks will also use the spiracles when not buried, and in some cases bidirectional flow (i.e., flow in and out of the spiracles rather than out of the branchial valves), has even been observed, perhaps to avoid disturbing the sediment around them. Exclusive use of spiracles for ventilation has only been observed in benthic species, but spiracles are present in most elasmobranchs, including both benthic and nonbenthic species. Aside from gill ventilation, spiracles may also be used to sample water passing by for chemosensation.
Gills and Gill Ventilation in Hagfishes
The gills of hagfishes are located within branchial pouches, which are posterior to the pharynx and are not supported by branchial arches (Figure 14.11). The branchial pouches have muscular outer walls and gill lamellae (folds) contained within. Like all other fishes, hagfishes have countercurrent gas exchange. Water flows unidirectionally, entering each branchial pouch through its afferent duct and exiting through its efferent duct, while blood flows through the gill lamella in the opposite direction. Gill ventilation is driven by the movement of the velum and peristaltic contractions of the branchial pouch walls. Unlike the velum of lamprey, in hagfishes, the velum is shaped like two curled sheets of paper, and it furls and unfurls to pump water through the head. Water is drawn in through the nostril or mouth, passes through the pharynx, is pumped into the afferent ducts and out of the efferent ducts of the branchial pouches, and then flows out of the external gill openings. Some species have one common opening on each side of the hagfish, and some have separate openings for each branchial pouch.
Lungs and Gas Bladders
Lungs and gas bladders are air-filled sacs within the body, found in lobe-finned and ray-finned fishes (Figure 14.14). Lungs are present in all lobe-finned fishes (lungfishes and tetrapods) as well as in some ray-finned fishes (specifically polypterid species). All other ray-finned fishes possess gas bladders (either respiratory or nonrespiratory). While nearly all lungs are well vascularized and used for respiratory gas exchange, the gas bladders of only some fishes are vascularized and used for respiration. These respiratory gas bladders connect to the mouth via a pneumatic duct (i.e., are physostomous) and are ventilated using air breaths, just like lungs. Nonrespiratory gas bladders are often called “swim bladders” and in most species lack a passage to the mouth (i.e., are physoclistous). In most air-breathing fishes, air breathing is used to supplement gill ventilation, either when dissolved oxygen is too low to meet their oxygen demand or when their oxygen demand is elevated (e.g., because of high physical activity) and cannot be met by gill ventilation. In some air-breathing fishes, air breathing is the primary source of oxygen, and the gills are reduced. These species typically live in habitats with prolonged hypoxia, and their reduced gills minimize the loss of oxygen to the water.
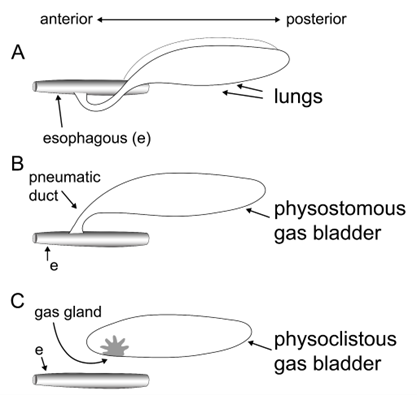
Figure 14.14—Basic anatomy of air-filled organs. (A) Lungs are paired air-filled organs. In nontetrapod fishes, the lungs connect to the esophagus (as illustrated here) via a pneumatic duct. In tetrapods, the lungs connect to the mouth via the trachea. (B) Physostomous gas bladders are unpaired air-filled organs that connect to the esophagus via a pneumatic duct. Physostomous gas bladders may be respiratory (i.e., vascular) or nonrespiratory. Some have a gas gland for buoyancy control. (C) Physoclistous gas bladders are unpaired air-filled organs that lack a connection to the esophagus. A gas gland is used to control volume and buoyancy via diffusion of gas into or out of the gas bladder.
Air Breathing: Ventilation of Lungs and Gas Bladders
Air-breathing fishes ventilate their lungs and gas bladders using buccal pumping. Unlike the dual pump for gill ventilation, the buccal pump only uses expansion and compression of the buccal chamber and creates tidal flow of air into and out of the lungs or gas bladder (Figure 14.5). While buccal pumping causes airflow during most of the air breath (including changes to buccal volume and lung / gas bladder inflation), deflation of the lung / gas bladder is driven by hydrostatic pressure on the body wall, as well as possible contributions from smooth muscle fibers in the walls of the lungs or gas bladder.
Air-breathing fishes use one of two air breath types, a two-stroke breath or a four-stroke breath, both of which utilize buccal pumping but differ in their sequences of movement and airflow (Figure 14.11). The “strokes” refer to the number of expansions and compressions of the buccal chamber, by analogy to two-stroke and four-stroke piston engines (see Section 14.4, Evolution of the Respiratory System).
One clade of ray-finned fishes, Polypteriformes, has evolved a mechanism for aspirating air rather than using buccal pumping. Aspiration occurs when lung ventilation is caused by active movement of the body wall and thereby the lung wall. This is in contrast to buccal pumping, where airflow is caused by active movement of the head. In Polypteriformes, contraction of muscles in the body wall compresses the lungs and causes expiration. It also deforms the interlocking scales of the fish (“scale-jacket”), which surround the body like a suit of armor. When the muscles relax, the scale-jacket recoils, reexpanding the lungs and causing air to be inspired through the open mouth. This is the only nontetrapod fish known to use aspiration. Notably, these fish still move their buccal chamber in the pattern of a typical four-stroke air breath, the breath type expected for these ray-finned fishes.
Accessory Air-Breathing Organs
We have focused most of our attention on air-breathing fishes that utilize lungs or respiratory gas bladders retained from the ancestral air-breathing species at the base of Osteichthyes. However, the majority of air-breathing fishes have nonrespiratory gas bladders and instead use a variety of other air-breathing organs (Figure 14.9). In other words, these fishes belong to lineages that ancestrally lost the ability to breathe air into their physoclistous gas bladders and have gone on to reevolve different forms of air breathing. These novel air-breathing organs can be classified as involving adaptations to the gills, the membranes lining the oropharyngeal cavity, the digestive tract, and the skin. Species with elaborated gill structures or highly vascularized buccal membranes take atmospheric air into their mouths and hold it there to enable gas exchange. Many catfish species use their digestive tract for respiration (Figure 14.12). Instead of using buccal pumping to move air into the gas bladder, they pump air into the esophagus. This air moves through the digestive tract, and oxygen diffuses across the highly vascularized epithelia. Last, some species supplement their oxygen uptake using their skin and may move onto land temporarily, exposing their moist skin to the greater concentration of oxygen that is in the atmosphere.
Amphibians
Cutaneous and Oropharyngeal Respiration
The amphibians (frogs, salamanders, and the enigmatic caecilians) are the only major tetrapod group that uses the skin as a major gas-exchange organ. In order to facilitate cutaneous gas exchange, amphibian skin is generally thin, moist, and well vascularized. Many amphibians have evolved intricate, unique skin structures hypothesized to increase surface area and thus respiratory capacity. In extreme cases, some amphibians have either reduced or totally lost lungs, most famously in the salamander family Plethodontidae, relying entirely on cutaneous forms of respiration.
Oropharyngeal respiration is a specific form of cutaneous respiration that takes place across the skin lining the mouth and pharynx. In order to perform this respiratory strategy, an animal must pull air into the mouth, but it does not require the active filling of a lung or other internal organ. Many amphibians, both lunged and lungless, perform buccal pulsing, quickly filling the mouth with air and emptying it to exchange gases across the oropharyngeal surfaces.
The trade-offs between water loss and gas exchange are fundamental to how respiratory anatomy has evolved. Cutaneous respiration is particularly problematic from this perspective, because the entire exterior surface is potentially exposed to drying factors, as opposed to more internal gills or lungs. If a frog was to exchange oxygen across the entire skin organ, that frog would dry out very quickly in any dry environment. Unsurprisingly then, many amphibians are restricted to cool, moist environments. However, those amphibian species that venture into drier, hotter environments have reduced cutaneous respiration, with thicker, drier skin that is less susceptible to water loss, and they rely more strongly on lung respiration.
Gills and Gill Ventilation
While lungs are the dominant respiratory structure across tetrapods, many amphibians retain some form of gills over the course of their development. In the prototypical life cycle of amphibians, an aquatic egg hatches into a larva with gills, which later transforms into a more terrestrial adult that lacks gills. This is true for many frogs, salamanders, and caecilians. However, some neotenic salamanders retain gills into adulthood, perhaps most famously in the axolotl (Ambystoma mexicanum), a highly endangered salamander native to central Mexico that is now common in the pet trade.
Amphibian gills generally fall into two groups: internal and external. Internal gills are contained within a chamber of the mouth, and a series of vascular gill tufts provide gas exchange when water is pumped into the mouth, through the gills, and out an opening or series of openings. External gills are outside the mouth, with large, branching tufts typically sitting directly behind the head. These gills are ventilated not by moving water through the mouth but instead by moving the head and/or moving the external gills through the water.
Frog tadpoles typically have internal gills, while salamanders with gills typically have both internal and external gills. In salamanders, the internal gills have a series of openings on either side of the head, so water pulled into the mouth can be pumped out in every direction. Frog tadpoles have a different system, where the gills empty into a single spiracle, typically on the left side of the body.
Lungs, Buccal Pumping, and Active Expiration
Even with vascular skin, and gills in some cases, lungs are still an important site of gas exchange for many amphibians. This is particularly true for oxygen uptake, as the skin and gills are more effective as CO2 eliminators. Amphibian lungs are generally unicameral: Each lung consists of a single primary chamber. This chamber is surrounded by a net of connective tissues including blood vessels and a capillary net for gas exchange as well as smooth muscle bands that squeeze the lungs into a honeycomb shape, increasing surface area.
Amphibian lung ventilation typically follows a two-stroke pattern of breathing that is similar to that seen in lungfish (Figure 14.11). Air breathing begins with oropharyngeal expansion, during which the lungs empty and fresh air is pulled into the mouth. Following this, a single compression of the oropharynx refills the lungs, completing a single breath. Unlike lungfish, however, some amphibians use axial musculature to empty the lungs.
Larval Lung Ventilation and Bubble Sucking
People often assume that gills and lungs do not overlap in the life cycle of frogs and that larval tadpoles use gills and then switch over to lungs at adulthood. In reality, however, many larval amphibians breathe air into the lungs at a very early age, in some cases even before feeding begins.
In order to breathe air at such small sizes, tadpoles must contend with the surface tension of water. Surface tension is a phenomenon created by extra hydrogen bonds between water molecules at the interface of water and air. For large animals, the surface tension of water is a trivial barrier to reaching the surface, and so whales and other aquatic animals easily breach the surface to breathe in air. However, breaking through the surface tension is a serious feat for smaller animals. Tadpoles deal with this problem by “bubble sucking,” a form of air breathing where instead of breaking through the surface, the surface is sucked down into the mouth as small bubbles and then bubbles of air are compressed into the lungs. As tadpoles grow larger, they eventually become large enough to breach the surface and so transition from bubble sucking to breach breathing (Figure 14.15).
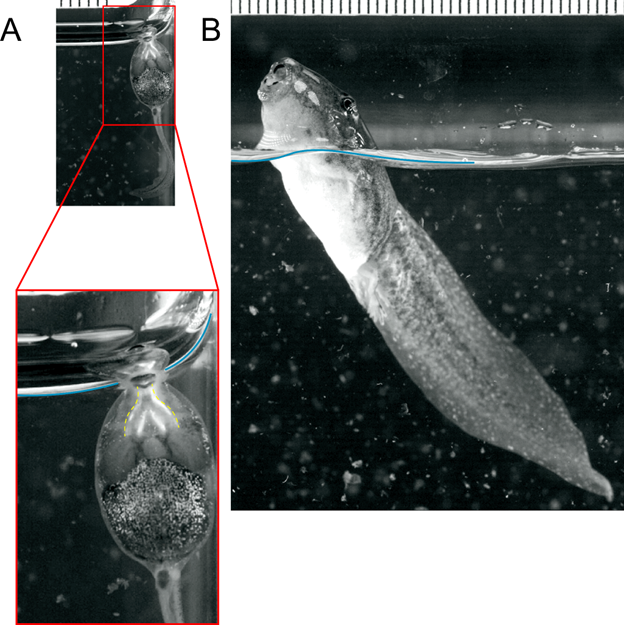
Figure 14.15—Bubble-sucking and breaching frog tadpoles. (A) A small Rana tadpole bubble sucks. Notice that the air bubble is sucked into the mouth (outlined in yellow in the inset) from the level of the surface tension (in blue). (B) A large Rana tadpole easily breaks through the surface, breaching to breathe air well above the surface. The two photos at the top share the same scale, showing the great increase in size over development. Stills taken from videos by Jackson R. Phillips and Kurt Schwenk.
Nonavian Reptiles
In addition to the diversity of lung anatomy and structural differences among nonavian reptiles, we will see that there are additional mechanisms for lung ventilation. We will tackle these structural and functional elements simultaneously, as it will help us better understand the linkage between structure and function if we can visualize the lungs ventilating along with the changes in their structural elements.
One of the largest differences between the lungs of many reptiles and amphibians is the subdivision of the lung into distinct large chambers (lobes, as we would refer to them in mammals). Amphibians generally have unicameral lungs—that is, each lung has one large chamber. Reptiles (and other amniotes) generally have multicameral lungs, meaning each individual lung may have multiple chambers (Figure 14.16). At a superficial level, the division of one lung into multiple chambers allows for increasing the surface area available for the diffusion of oxygen and carbon dioxide. As reptiles (and other amniotes) evolved into larger sizes and some became more active, the additional surface area helped support the additional energy requirements. Whether air moves from one chamber to another is highly dependent on species and stage of ventilation.
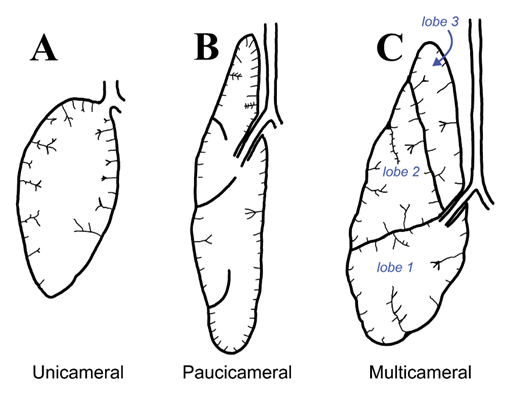
Figure 14.16—The three different lung types found in reptiles. (A) Unicameral lungs have a single chamber, surrounded by folds that increase surface area and carry vasculature. (B) Paucicameral lungs have larger divisions between different areas of the lung, further increasing surface area and topographical complexity. (C) Multicameral lungs have full divisions between different lung lobes and have high surface area and functional complexity.
The lizards will serve as a convenient starting point for investigating changes in both the structure of the lungs and their ventilation. Partially, this is because lizard lungs are extremely variable across different lineages. Some lizard lungs are unicameral like the amphibians, some are multicameral with distinct chambers, and finally some are paucicameral (incomplete separation of lung chambers; Figure 14.16). To further complicate the issue, it is difficult to pull apart trends within lizard groups as to who has which type of lung. The monitor lizards (Varanidae) have complex multicameral lungs, while their neighbors on the phylogenetic tree have both unicameral lungs (tegus, Teiidae) and paucicameral lungs (iguana, Iguanidae). Even within a group like the monitor lizards, lung complexity is very different, especially if you are comparing a Komodo dragon to a Savannah monitor.
With great variation in complexity comes great variation in behavior. There are multiple mechanisms of ventilation in lizards. The most common of these is known as costal aspiration. Costal refers to the ribs, which in this case are the main drivers of ventilation. Remember that the lungs are ventilated via changes in pressure. In the amphibians, positive pressure in the buccal cavity pushes air into the lungs. The lizards are our first example of using negative pressure to draw air into the lungs rather than push it. In costal aspiration, the ribs rotate outward (we are simplifying the motions), increasing the volume of the body cavity. Increasing volume decreases the pressure, and air is pulled into the lungs to balance the pressure. To exhale, the muscles that rotated the ribs can relax, and as the ribs return to their original location, the positive pressure they generate helps push the air back out of the lungs. However, if you have ever seen a lizard run, you know that they bend their bodies quite a bit side to side while they move. That lateral bending also involves active control of the ribs (and obviously the vertebral column), so how do lizards breathe and move at the same time? For many lizards the answer is simple; they do not. Many species will simply hold their breath while they are running, necessitating that they must stop periodically to quite literally catch their breath. However, there are other methods that some lizards can employ. Smaller lizards, like the anoles, can still make use of buccal and gular pumping to push air into the lungs, particularly after quick bouts of intense locomotion. Larger lizards, like the monitor lizards, have increased amounts of smooth muscle associated with the lungs, hypothesized to help move air through the lungs.
We have described a fair bit of variation in the morphology of lizard lungs while neglecting one very large group of lizards: the snakes. This is intentional, as snakes represent a distinct change in body plan that impacts many different body systems. Like many of the other organ systems in snakes (and snakes themselves), the respiratory tract becomes quite elongated in snakes. There are two key elements, one structural and one functional, that make snakes unique. In the most recently diverging groups of snakes—the Colubridae, Elapidae, and Viperidae, among others—there are technically two lungs, but one becomes highly reduced during development and in many cases disappears. In all these groups, it is the left lung that is highly reduced or has vanished. The earlier diverging snake groups (the boas and pythons, for example) retain two individual lungs. In the elongated lungs, there is also evidence that the entire lung is not used for respiration. The most posterior section of the lung is often referred to as the saccular portion of the lungs, while the more anterior sections are the respiratory portion. How can you tell the difference? The saccular portion lacks blood vessels associated with the lung itself, making it impossible for gas exchange to take place. Additionally, it appears that at least a few species of snakes can breathe using different regions of the lung independently. For example, boa snakes are able to breathe with different parts of the lung during prey constriction to ensure they are able to both squeeze their prey and ventilate their lungs.
Moving to the turtles, some of our base assumptions for how reptiles breathe need to be updated. This is primarily because of the change to the ribs in turtles (see Chapter 9—Postcranial Axial Skeleton). The ribs, becoming fused to and playing a vital role in the structure of the shell, are no longer mobile enough to enable costal aspiration. So how do turtles create the pressure differences needed to move air into and out of the lungs? There are mobile elements of the skeletal system of turtles: The limbs and some of the abdominal muscles can be manipulated. The muscles associated with the limbs and the abdominals function to create the same pressure changes we saw in the lizards. However, the shell limits the mobility of these elements. So how is the pressure created? In turtles, we see that if the skeletal body wall cannot be moved, then the organs themselves must move. By moving the viscera within the body cavity, resulting pressure changes allow for the lungs to expand and contract.
Turtles are also famous (infamous?) for developing an additional method for breathing. Many turtles spend their winters buried in the mud of ponds and rivers, awaiting the warmth of spring. However, this means that they are unable to breathe with their lungs during their long submerged slumber. While their metabolic rates drop precipitously, they do not completely shut down and still require oxygen. Their alternative method of respiration involves gas exchange through their cloaca. Turtles have a highly vascularized cloaca, with a thin layer of tissue separating the blood vessels from the external environment. This use of cloacal ventilation provides enough oxygen to meet all the turtle’s metabolic needs during the winter until it can resume using its lungs in the spring.
The crocodylians, to no surprise, also have a few anatomical innovations for their respiratory system. In many ways, we can point back to what we observed in the lizards (and in particular the monitor lizards) for some of the structures and behaviors present in the crocodylians. Crocodylians have multicameral lungs, with extensive subdivisions and highly complex surfaces for increasing surface area. However, crocodylians possess a muscle that is not found in other vertebrates and is used for ventilation. Running from the pelvis to the liver is a muscle known as the diaphragmaticus (Figure 14.17). Convergent to the mammalian diaphragm, the independently evolved diaphragmaticus works in a very similar but subtly different way in the crocodylians. The diaphragmaticus attaches posteriorly to the liver (and other structures). The liver is physically connected to the lungs. When the diaphragmaticus contracts, the liver and lungs are pulled posteriorly. This creates the negative pressure to pull air into the lungs, and when it relaxes, the positive pressure created by the liver returning to its resting position pushes the air back out. We refer to this as the hepatic piston, moving the liver back and forth in such a way to change the pressures.
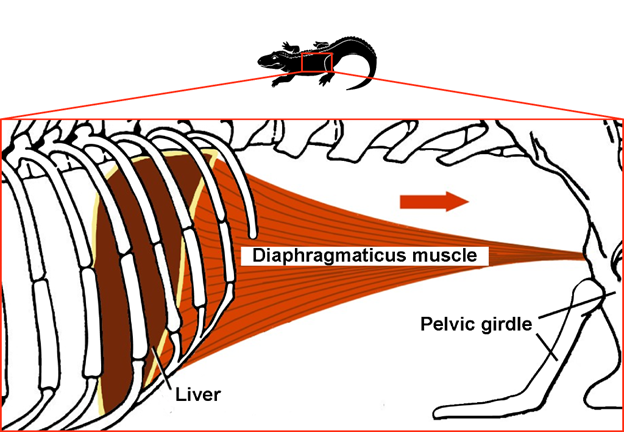
Figure 14.17—The diaphragmaticus muscle in crocodylians. Contraction of the diaphragmaticus muscle (red arrow) pulls the liver posteriorly and expands the lungs (which are anterior to the liver), which causes inspiration. Relaxation of the diaphragmaticus and contraction of abdominal muscles moves the liver anteriorly and compresses the lungs, causing expiration.
Aves
When considering the respiratory system of the birds, it is helpful to remind ourselves of two important features of bird behavior and physiology that impact the respiratory system. The first is that most birds undertake powered flight. Filling the body with air instead of water may help with flight, reducing the density of an individual. The second is that birds are endothermic, which comes with an increased metabolic rate. If more energy is needed to maintain a stable internal body temperature, then more oxygen is required to make use of that energy. It should come as no surprise then that the avian respiratory system is quite effective at obtaining oxygen. Importantly, birds have unidirectional airflow throughout their respiratory tract, which promotes efficient gas exchange by maintaining larger concentration gradients, as we discussed earlier in this chapter.
To begin, we should define some new characters. Birds possess all the major structural elements of vertebrate respiratory systems: larynx, trachea, bronchi, and lungs. Birds also possess a syrinx, a structure that allows for the production of sound, as well as a series of structures known as air sacs. The air sacs are not directly involved in gas exchange; instead, they play a more complex role in the movement of air throughout the respiratory system of birds. The number of air sacs and their position in the respiratory system is quite variable, with new discoveries being made continuously (in fact, as the authors write this, a new paper came out in Nature on a type of air sac in soaring birds). Let us use a simplified model to illustrate how the air sacs work in conjunction with the other elements of the respiratory system to visualize how birds generate the unidirectional airflow (Figure 14.18).
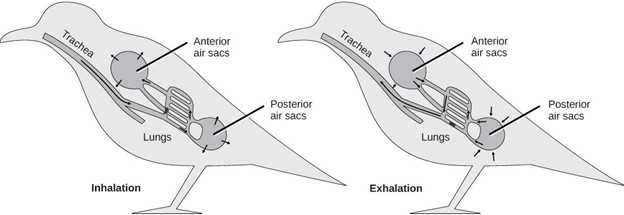
Figure 14.18—Simplified anatomy of the avian respiratory system. Two cycles of expansion and compression of the air sacs must occur in order to move a parcel of air through the entire respiratory system. The first inhalation draws air through the trachea and bronchi into the posterior air sacs and lungs. The first exhalation causes that air to move from the posterior air sacs into the lungs. The second inhalation draws air from the lungs into the anterior air sacs. Last, the second exhalation expels air from the anterior air sacs out through the bronchi and trachea.
In our model as presented in Figure 14.18, there are two air sacs: anterior and posterior. The air sacs help separate the functional roles of ventilation and gas exchange. In nonavian reptiles, the lungs have to be stretched open, creating the negative pressure that moves air into the lungs. In birds, the lungs no longer have to be flexible and elastic because the air sacs can be stretched instead. The air sacs are completely avascular, and their entire role is to act as “bellows,” expanding and contracting to push air through the lungs. The expansion and contraction of the air sacs are accomplished through movement of the ribs, another example of costal aspiration as we observed in nonavian reptiles. Additionally, many birds can move their sternum a significant amount, enough to also generate pressure changes and the movement of air. In contrast to the air sacs, the lungs themselves are relatively rigid, not expanding or contracting as air is moved through them. This allows the lung tissue to be more specialized for gas exchange without the constraint of needing to be flexible for ventilation. Specifically, the lung tissue between the blood capillaries and the air can be extremely thin because this tissue does not need to stretch. Air moving through the lungs passes first through parabronchi, then narrow passageways known as air capillaries. The walls of the air capillaries are extremely thin, much thinner than that of the other gas-exchange surfaces in vertebrates. If this was not enough, the orientation of the blood vessels also increases the efficiency of gas exchange. Recall that earlier we discussed crosscurrent exchange, where blood moves at a perpendicular angle to the air, which creates an increased oxygen concentration gradient between the air and the blood (Figure 14.3).
How does this complex system ensure one-way airflow through the whole tract? Let us follow one “air molecule” as it makes its way through the system, using Figure 14.18’s simplified tract. Note that it is going to take two full breaths to go from start to finish for this air molecule, and we will have to refer to stages like inhalation 1 and inhalation 2. During inhalation 1, one air molecule enters the nares, passes through the trachea and bronchi, and travels into the posterior air sacs. Not all of the air travels into the air sacs; some will move directly into the lungs. During exhalation 1, the air molecule is pushed out of the posterior air sacs and travels through the parabronchi in the lungs. During inhalation 2, it moves from the lungs into the anterior air sacs. Finally, it is during exhalation 2 that the air molecule is pushed from the anterior air sacs back into the trachea and out of the body.
The adaptations of bird lungs, including extremely thin blood-gas barriers, crosscurrent exchange, and unidirectional airflow, create an incredibly efficient system of extracting oxygen from the air. It accommodates the intense oxygen requirements of endothermy and flight. In fact, it has allowed birds to take flight to some extremes previously thought impossible. Bar-headed geese have been observed flying over Mt. Everest, over 8 km above sea level. The availability of oxygen at that height was thought to be too low to support any animal, but the bar-headed goose can simply cruise over Mt. Everest thanks to its respiratory system and some physiological tricks.
Box 14.2—Scientist Spotlight: Colleen Farmer and the Evolution of Unidirectional Airflow in Reptilians
Birds have perhaps the most bizarre lungs among all vertebrates. For as long as humans have interacted with birds, we have known that bird lungs are attached to strange sacs that extend around the body, unlike any other group of animals. These structures were first formally described in 1573 by Volcher Coiter, an important figure in the field of comparative anatomy who was one of the first Europeans to study comparative osteology.
Today, we understand that air sacs in bird lungs have several functions, but one dominant function is facilitating unidirectional airflow through the lungs. The first experiments to demonstrate unidirectional airflow in bird lungs used powdered charcoal to track the passage of air through the bird respiratory system. Exactly how unidirectional breathing was accomplished was hotly debated at the time, with some scientists suggesting that some valve preventing backflow must be present in order to explain the one-way flow of air through the lungs. The modern understanding of bird lung ventilation was established by Dr. E. H. Hazelhoff, who advanced a hydrodynamic theory of airflow in the bird lung, suggesting that a combination of looped passages and air sacs produce unidirectional airflow in birds without needing any valves. After this breakthrough, the following 70 years saw hundreds of papers published on bird lungs and their unidirectional flow. Because the complex air sacs and looped passages are only seen in bird lungs, it was a foregone conclusion that unidirectional airflow was unique to birds.
Unidirectional airflow has many advantages, because it allows fresh, oxygenated air to pass continuously across a vascular surface. This continuous flow allows for crosscurrent gas exchange, increasing the rate of oxygen uptake to very high levels. Unidirectional air breathing and crosscurrent exchange were originally thought to have uniquely evolved in birds to support the high metabolism required for endothermy and powered flight. This paradigm was intuitive and unchallenged for over 100 years, until Dr. Colleen Farmer of the University of Utah totally changed the game.
Dr. Farmer made the first crack in the armor in 2011 when she published a paper describing unidirectional airflow in the lungs of the American alligator (Alligator mississippiensis), a crocodylian, in the journal Science. Crocodylians are decidedly not a flying species and are not even endotherms, so the presence of unidirectional flow seemed totally out of place. However, crocodylians are relatively closely related to birds, with the two together forming the living archosaurs. Dr. Farmer suggested that unidirectional air breathing was an ancestral trait of archosaurs that was likely also shared by extinct members like the dinosaurs and pterosaurs.
The literature has not stopped there, either. Two members of Dr. Farmer’s lab, Drs. Emma Schachner and Bob Cieri, went on to describe unidirectional airflow in a series of lizards. They found that multiple unrelated lizard groups, including monitor lizards and iguanas, all have unidirectional airflow in the lungs, pushing the hypothesized origin of unidirectional air breathing all the way back to the origin of reptilian tetrapods. Finding unidirectional airflow in iguanas was especially surprising, given their relatively simple, unicameral lungs. These findings have revolutionized the field and opened up many new hypotheses and possibilities for how, when, and why different types of air breathing have evolved.
So often in science, a good, reasonable hypothesis is slain with a bit of investigation. Fifty years ago, no biologist would have thought that an iguana lizard could possibly have unidirectional airflow in the lungs. However, as the evidence builds, it would hardly be surprising if any reptile was found to have unidirectional airflow in its lungs today.
Mammals
The mammalian lung is unique in many ways but in some ways requires us to go back to where we began, with the nonavian reptile lungs. Mammals have biphasic, tidal breathing (one inhalation, one exhalation), where air enters and leaves through the same pathway. Mammalian lungs are multicameral, with completely separated lobes. The number of lobes varies in mammals and even within an individual. For example, dogs have four lobes in the right lung and two in the left. Humans have three on the right and two on the left (Figure 14.19).
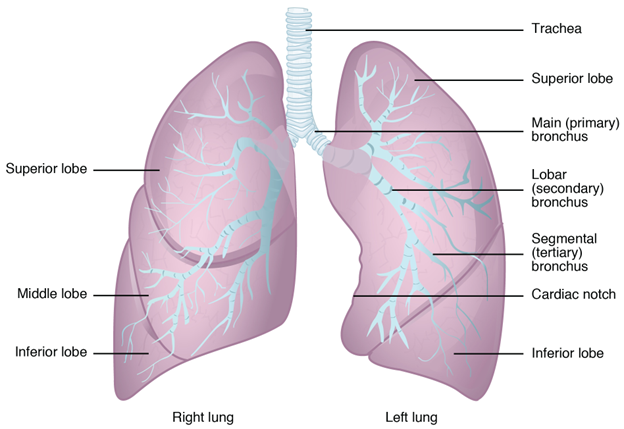
Figure 14.19—Human lungs with lobes labeled. The right lung has three lobes (superior, middle, and inferior). The left lung has two lobes (superior and inferior) and accommodates the heart, which is located slightly left of midline.
The lungs themselves are surprisingly dense compared to other groups. In the section on birds, we discussed how air travels through progressively narrower passageways (from parabronchi to air capillaries), which increases the amount of surface area available for gas exchange. Mammals will take a different approach with a similar outcome. In mammals, the bronchi divide into bronchioles. These bronchioles will continue to divide into smaller and smaller channels for air to pass through. However, unlike in the birds, where the channels become air capillaries, the passages dead-end in mammals. They dead-end in areas we refer to as alveolar sacs. The alveolar sacs appear as extremely bumpy balloons, but each bump can be considered an alveolus (Figure 14.3). The alveolus is where gas exchange occurs in mammals. Each alveolus is completely wrapped in capillaries, minimizing the distance between the inhaled air and the blood supply. The lung is completely full of alveoli, making use of every possible space to dramatically increase the surface area available for gas exchange to occur. How many alveoli are there? Estimates in humans range, but recent work has put the number at 480 million alveoli in the average person, with some estimates at almost 800 million. While the movement of air through mammalian lungs may be not as efficient as we observe in the birds, the incredible increase in surface area available for gas exchange allows the mammals to be endothermic and highly active.
While mammalian lungs use bidirectional flow for moving air in and out of the lungs, the manner in which they do so is unique among vertebrates. Mammals have the ability to alter the volume of air coming into/out of the lungs with any particular breath. Your “normal” breathing, the standard in and out that you often don’t think about, is what we refer to as tidal breathing. It moves in and out of its own accord, like the tides. The ventilation of tidal breathing is largely done by the diaphragm. The diaphragm is a skeletal muscle that sits between the liver/stomach and the lungs. The diaphragm, at rest, is shaped like a hill, with its highest point in the center and the edges much more inferior. The edges are connected to the last few ribs, thoracic vertebrae, and the abdominal body wall. At its center, a central tendon connects the diaphragm to the pericardium, the membrane surrounding the heart. When the diaphragm contracts, it pulls downward/inferiorly, increasing the volume of the thoracic cavity, thereby reducing the pressure and causing the lungs to fill with air (Figure 14.20). In order to exhale, the diaphragm relaxes, and the elasticity of the lungs pulls the diaphragm to its previous position and pushes the air outward under positive pressure.
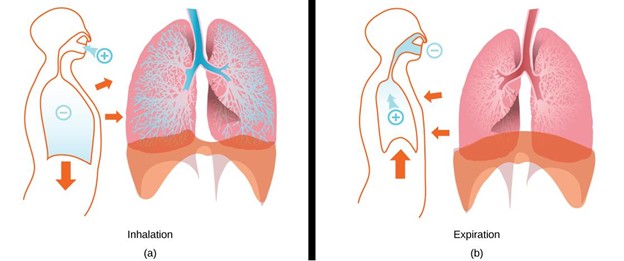
Figure 14.20—The mechanics of lung ventilation in humans. (A) During inhalation, the diaphragm and intercostal muscles contract, expanding the pleural cavity and the lungs. This draws air into the lungs through the mouth or nose. (B) During exhalation, the diaphragm relaxes, allowing the lungs to elastically reduce in size and causing air to be exhaled.
However, mammals can do more than just tidal breathing. Mammals can consciously take very large breaths, for inhalation as well as exhalation. To accomplish this, they must involve other structures. The volume that would be displaced by tidal breathing is still done by the diaphragm. To increase the volume of the thoracic cavity even further, mammals incorporate costal aspiration as well. The ribs, while less individually mobile than they are in the reptiles, rotate (as an entire unit) outwards and cranially. This further increases the volume of the thoracic cavity, allowing for the intake of even more air.
14.6 Integration
Like everything in the body, the respiratory system does not and cannot exist in isolation. In humans, oxygen brought in by our lungs must go somewhere, and the carbon dioxide expired must come from somewhere. Effective respiratory organs also need input from our central nervous systems to ensure we keep breathing (even when we are asleep!). The respiratory system is also integrated with other systems from an evolutionary perspective. Evolutionary changes in other organs must accommodate the respiratory system and vice versa. Due to the critical nature of respiratory function, these effects can be quite powerful in shaping the evolutionary history of lineages. The integration of the respiratory system is central to understanding its evolutionary history and functional role in animals today.
The Path of Oxygen: Connecting the Respiratory and Cardiovascular Systems
Nearly every molecule of oxygen gas used by any Earth-borne, vertebrate animal, was originally formed by some form of natural photosynthesis and released into the atmosphere. That oxygen must then diffuse into the bodies of vertebrates to be used, typically through the skin, gills, or lungs. Dissolved oxygen is then transported by the bloodstream to a cell in need, where it is used in cellular respiration. Vertebrate blood has special oxygen-carrying ferroproteins (iron-proteins), which bind oxygen and carry it around the body. Eventually, most of that oxygen diffuses into mitochondria, which use oxygen to convert macromolecules like glucose into cellular energy (ATP) that powers an animal’s metabolism. During that process, the by-product carbon dioxide is created, which then diffuses back into the bloodstream. When dissolved in blood, carbon dioxide converts into carbonic acid, acidifying blood and creating a potential problem (our bodies cannot operate with strongly acidic blood). To deal with this problem, our blood carries carbon dioxide to the lungs, where it diffuses out into the air we exhale. The cardiovascular system enables the respiratory system to function at a basic level, and the two are deeply and fundamentally integrated.
Breathing Control: Nervous Control of Respiration
When you actively think about breathing air, you can easily control how quickly and deeply you breathe. However, during and after exercise or in stressful moments, controlling your breathing can be very difficult. On the other hand, you breathe constantly without being aware of doing so, and you breathe every night while sleeping without any active input. The respiratory center in your brain is located in the brain stem. This brain region is ancient and deeply conserved across vertebrate taxa. The respiratory center controls air breathing, allowing you to breathe without thinking about it, but it also adjusts respiratory rates to environmental signals.
One obvious way in which our nervous systems adjust breathing rates is during exercise. When we exercise, more cellular energy is used in our cells, requiring more oxygen and creating more CO2, acidifying our blood. Our body and brain notice this change, and one of the first responses is to increase breathing rates, allowing us to expire more CO2 and inspire more O2. The same thing happens at high elevation, where there is less oxygen in the air and our brains respond by unconsciously increasing breathing rates. The brain also responds to stress by increasing breathing rates, presumably to prepare for a stressful event, but in many parts of the world today, this response can be maladaptive, as our stressors tend to be less “saber-toothed tiger” and more “homework due on Monday.”
In nonhuman animals, similar or identical systems also exist. For many air-breathing fishes and aquatic amphibians, air breathing is facultative (while gill ventilation remains obligatory). This means that many species rarely breathe air normally but significantly increase air-breathing rates when environmental oxygen levels fall or when physiological demand for oxygen increases (such as during a high level of activity). Similarly, hyperoxygenation of the environment lowers rates of air breathing for many species. While the nervous system orchestrates the actions of the respiratory system, the respiratory system in turn provides the oxygen needed to generate nerve impulses in the first place.
Box 14.3—Integration Breathing Activity: With a Partner, Learn How to Take Basic Vital Signs and Understand How We Can and Cannot Control Our Respiratory Biology
First Thing: Baseline Vitals
Two of the most important vital signs are also the easiest to learn how to measure: the pulse and breathing rate. The pulse is a rhythmic measurement associated with the speed of contractions in the beating heart. This beat radiates out as a “pulse” across the body, and so any major artery in the body has a pulse. The two most common places to measure a pulse are the radial artery in your wrist and the carotid artery in your neck.
Figure 14.21—How to take a radial pulse using three fingers to press firmly on the radial artery, which runs over the thumb side of your lower forearm and wrist.
To begin, place your three middle fingers on your own wrist just below the thumb, as shown in Figure 14.21. With a firm pressure, move your fingers around slightly until you can feel the pulse, increasing pressure if necessary. If you are having difficulty, you can try the carotid artery instead, feeling on the side of your neck just next to your esophagus with two fingers. Once you can easily find your own pulse, obtain permission from your partner to try to find their pulse as well. To measure a pulse, set a timer for 30 seconds and then count the number of beats during that time period. To get the number of beats per minute, multiply your number by two. Write that number down in a format like Table 14.1.
Next, practice measuring breaths. This is probably easy to do for yourself, but because we can consciously control our breathing, it is usually best to have someone else measure your breaths. Try watching your partner’s shoulders or back rise and fall to count the number of breaths. Once again, count the number of breaths for 30 seconds and multiply by two to get the number of breaths per minute, and then add the value to the first column of Table 14.1.
Table 14.1—Experimental data of your own breathing
|
|
Baseline |
Experiment 1 |
Experiment 2 |
|
Pulse (beats/min) |
|
|
|
|
Breathing rate (breaths/min) |
|
|
|
Experiment 1: What Can We Control?
You may not be able to consciously contract your heart to beat whenever you want, but you can behaviorally control your breathing in most scenarios. For this experiment, we will see whether we can indirectly control our hearts using our lungs.
For two minutes (or longer), try to actively slow down your breathing. Only go as far as is comfortable, and do not hold your breath. Instead, try to make your inhalations and exhalations longer. Use your diaphragm to fill your lungs all the way up, then slowly let all the air go. It can be helpful to consider all the information you have learned about the respiratory system to this point, and think about the flow of air, blood, and oxygen that is allowing you to even perform this little exercise.
Once you have reached a consistent rate of breathing for at least a minute, have your partner measure your pulse and breathing rates as described above. Enter those values into Table 14.1, then decide whether you would like to return to the activity or take another few minutes to just breathe. Do you feel different after this experiment? In what ways?
Experiment 2: What Can’t We Control?
A different form of respiratory control is physiological rather than behavioral. Here we will simulate a high-stress event using physical exercise. If you have a medical condition that makes exercise dangerous, please consider skipping this activity. Otherwise, pick your favorite form of exercise, such as jumping jacks, push-ups, running down a hallway, or going up and down stairs. Do one or more of these activities vigorously for at least two minutes and then have your partner help measure your vitals as quickly as possible. If you can, measure your own pulse while your partner measures your breathing rate and enter them into Table 14.1.
Follow-Up Questions
- In experiment 1, you actively slowed down your breathing. Did your pulse also change with your breathing? What organ systems are being integrated to form that response?
- After exercising in experiment 2, what happened to your pulse and breathing rate?
- Why are these responses important? What is happening at the cellular level during exercise that makes these responses critical?
Connections Between Ventilation and Other Behaviors
Ventilation, or the active process of breathing, is shared among nearly all vertebrates. There are differences, of course, as fish and frog tadpoles ventilate their gills with water, while lizards, lungfish, and lions ventilate their lungs with air. However, what all these animals share is that ventilation happens through the mouth (though many tetrapods use their nostrils as well). Water or air enters the mouth and then proceeds to a respiratory organ of some kind. In fishes, gill ventilation behavior is nearly always produced by some form of oropharyngeal pump. During inhalation, the mouth opens and the interior pharynx expands in volume, drawing water in. During exhalation, the mouth closes and the pharynx compresses, squeezing water out through the gills. For the oropharyngeal pump to work, fish mouths need to be able to perform these actions. Of course, fish mouths also have a lot of other jobs, like eating. Over evolutionary time, fish mouths have evolved many amazing shapes and forms for different feeding strategies, but all these forms are constrained by the need to continuously pump water over the gills.
In terrestrial amphibians, the buccal pump is used for air breathing, and this pump is powered by movements of the hyobranchial apparatus, including the hyoid bone. This set of bones in the throat is also critical for tongue movements. An interesting trend across salamanders is that species with reduced or absent lungs often have strongly divergent feeding modes and hyoid shapes not seen in salamanders with large lungs. It has been hypothesized that the evolutionary loss of lungs in salamanders allowed for the hyoid to evolve in new ways because the buccal pump was no longer needed.
A similar story has been told to describe the situation for amniotes, which made the transition from the buccal pump to aspiration breathing for lung ventilation (see Section 14.4, Evolution of the Respiratory System). Many reptiles use costal respiration to ventilate the lungs, but these same species also rely on the ribs for flexion of the body wall during locomotion. For many species, the ribs cannot be used for breathing and running at the same time. This is a big potential problem, because running is an energy-intensive activity where carbon dioxide elimination and oxygen uptake are critically important. Many lizards deal with this by optimizing their use of anaerobic metabolic pathways, which is a way to generate cellular ATP without oxygen. Anaerobic metabolism results in lactic acid buildup, and many lizards are extremely tolerant of lactic acid, often resulting in amounts that would be devastating to a typical mammal.
Mammals took a different evolutionary route than lizards and other reptiles. Mammal ancestors likely also relied on costal ventilation but eventually transitioned to a muscular diaphragm, which decoupled locomotion from air breathing. Many mammals, including humans, are particularly good at sustained activity via aerobic metabolism, as they can continuously ventilate the lungs during locomotion, providing sustained oxygen to muscle cells and quickly eliminating carbon dioxide.
Breathing air is a powerful route to bringing oxygen into the body. A necessary side effect of breathing air is that the addition of a large volume of air inside a body results in a change in buoyancy—air is less dense than all solid materials, including animal bodies. In some cases, this is useful, as we see for many fish that use swim bladders to achieve neutral buoyancy and improve swimming efficiency. Indeed, many animals adjust the volume of gas in their lungs or gas bladders to achieve different buoyancy similar to how scuba divers adjust their buoyancy control devices (BCDs) to stay neutrally buoyant. Some fishes have even evolved physoclistous swim bladders with no respiratory role (Figure 14.14). These bladders can be inflated and deflated to maintain neutral buoyancy without trips to the surface using a “gas gland” with a complex series of blood vessels (called a rete mirabile) set up for countercurrent exchange to fill the bladder with nitrogen gas. In physostomous species, the pneumatic duct (connection between the swim bladder and the mouth) is retained. Some physostomous species control buoyancy using a gas gland (like physoclistous species), some use air breaths (i.e., either release gas through the pneumatic duct or take in additional air from the surface and pump it into the gas bladder), and some may use both. Physostomous air-breathing fish also use air breaths to regulate their buoyancy. In other aquatic animals, sometimes negative buoyancy is useful. Crocodylians are thought to swallow large stones (gastroliths, literally “stomach stones”) at least in part to allow for easier buoyancy control and to stay at the bottom of water even with the lungs partially full of air.
Box 14.4—The Evolution of Vocalization in Vertebrates
The evolution of air breathing and lungs in vertebrates enabled the subsequent evolution of vocalization in tetrapods. The birds you hear chirping in the morning, the dogs barking down the street, the lecture your professor gave today, and even the text on this page (a representation of spoken words) can all be traced back to the evolution of air breathing. In the evolution of vocal communication, ventilatory airflow that originally served a respiratory role became co-opted for communication. Unlike water, it does not take much energy to vibrate air (i.e., produce sound), and the larynx and syrinx of tetrapods are adapted to produce and modify these vibrations.
In its simplest form, the glottis is the opening between the oropharyngeal cavity and the airways (and lungs). In lungfish, the glottis functions as a valve: It is a slit in the floor of the pharynx that is closed and opened by a sphincter muscle. In tetrapods, the glottis became more robust. Tetrapods evolved three cartilages (a pair of lateral arytenoid cartilages and a ring-shaped cricoid cartilage) that support the glottis. Muscles control the movement of the arytenoid cartilages, allowing tetrapods to actively open and close their glottises at will. Inferior to the glottis and larynx, reptiles (including birds) and mammals possess additional rings of cartilages around the airway, called a trachea.
Larynxes are diverse across tetrapods. Some lineages possess vocal cords, which are tissues attached to the arytenoid cartilages that vibrate when they are close to each other and air flows past them. Movement of the arytenoid cartilages modulates the pitch of the vibrations. While frogs are known for the sounds they make with their vocal cords (and vocal sac, as we will discuss next), all other amphibians, as well as most nonavian reptiles, lack vocal cords. In these cordless species, some still vocalize by rapidly expelling air from the lungs. Of course, mammals have vocal cords, and the sounds produced when the cords vibrate are modified or “shaped” by the position and movements of structures in the oropharynx, allowing for the great vocal flexibility observed in many mammals. Some mammals, such as red deer stags, even lower the position of the larynx in order to lengthen the vocal tract and alter the quality of their calls.
The characteristic croaking and chirping of frogs are possible thanks to both their vocal cords and vocal sacs. While all frogs have vocal cords, typically only testes-bearing frogs have resonating vocal sacs and produce mating calls. The vocal sac is an elastic chamber beneath the floor of the buccal cavity. To prepare to call, a frog inspires extra air into the lungs, then contracts muscles in the body wall (with its nares closed) to transfer gas back into the buccal cavity, where it flows into the air sac via slits in the buccal floor. These slits are closed by muscle around it. Now that the air sac is inflated, the frog starts to call: The frog cycles gas between the lungs and the buccal cavity, and as gas flows past the vocal cords, they vibrate. These vibrations are amplified by the inflated, resonating vocal sac.
Birds are renowned for their musical sound production, but they do not vocalize with their larynx. Instead, they have a syrinx, a structure located at the inferior end of the trachea, where the trachea splits into two bronchi. The cartilaginous rings in this region are specialized, and drum-like membranes between the rings (in the walls of the syrinx) vibrate as air passes. Sound characteristics are affected by muscles that modulate the tension of the membranes, by the shape of the trachea and oropharyngeal cavity, and by tongue movements. In some birds, the syrinx only spans the inferior end of the trachea, while in others the syrinx only spans the superior ends of the bronchi, and in most birds (including songbirds), the syrinx spans both the trachea and the bronchi. This anatomical complexity allows birds to simultaneously produce different sounds in different regions of the syrinx (e.g., membranes in the left and right bronchi), creating the musical complexity we know and love.
14.7 Human Respiratory System
Our lungs have the same characteristics that are shared by the lungs of other mammals. Our airways branch over and over again, ending in hundreds of millions of alveoli. Because of surface tension, these moist spherical structures (~0.2 mm diameter) want to deflate like little balloons. To reduce their inward pull, our lungs are coated in surfactant. When we are relaxed, we contract our diaphragm and intercostal muscles to inhale, and we exhale passively by relaxing our muscles and letting our alveoli pull inward. When we are breathing heavily, we recruit our abdominal and intercostal muscles to help with exhalation. The large cumulative surface area of our alveoli supports our high metabolic load, which results from being endothermic and having large, energy-hungry brains. Our gas-exchange capacity is larger than we typically need, allowing us to endure more extreme conditions, such as exercising at high altitudes or surviving disease. In this section, we will focus on the intersection between respiratory anatomy and human health.
Segments
Our airways bifurcate approximately 23 times (“generations”) before becoming alveoli. Arteries travel and branch along with the airways, matching the structure of the respiratory tree (Figure 14.22). This may sound like a trivial fun fact, but it has medical importance. If a portion of the lungs becomes diseased (e.g., becomes cancerous), then that portion can be removed without affecting arterial blood flow to other portions of the lungs. This procedure is called a segmentectomy or a lobectomy, depending on how large a portion is resected from the lung.
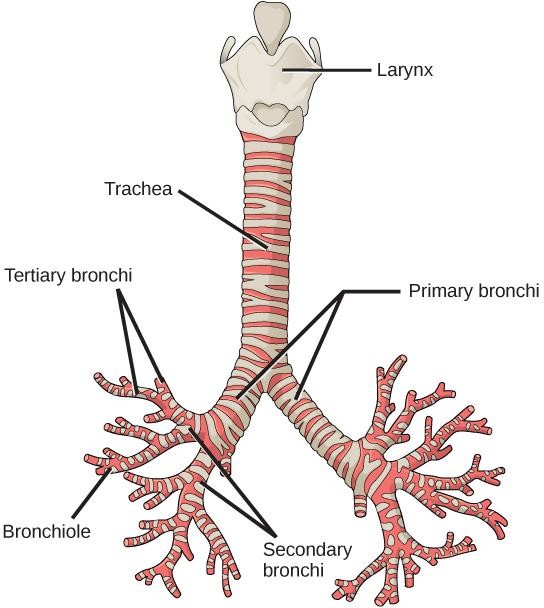
Figure 14.22—The branching pattern of the airways in the human lungs. On average, human airways have approximately 23 branching generations. The branching of the pulmonary arteries closely matches the branching of the airways. The left primary bronchus is oriented more horizontally than the right primary bronchus because the left bronchus passes over the heart.
Surfactant
At first glance, it may seem like only small organisms, like the tadpoles discussed in Section 14.5, Diversity of the Respiratory System, meaningfully interact with the forces of surface tension, not large organisms like us. However, surface tension is also an important force in the context of our respiration. Alveoli must remain moist to permit gas exchange, and the surface tension of this thin layer of water adds up to a large inward force. This force is useful because it drives exhalation, along with the elasticity of the lung wall. However, it also makes it more difficult to inhale. Surfactants are molecules that reduce surface tension, and they reduce how much force our muscles must produce when we take a breath.
Unfortunately, fetal surfactant production matures during the last 10 weeks (weeks 30–40) of gestation. Infants born before 34 weeks of gestation often develop respiratory distress syndrome (RDS) because there is not enough surfactant in their lungs. In these cases, infants have difficulty breathing because of the extra work to resist surface tension and because their alveoli may collapse. RDS is treated by administering artificial surfactant, providing breathing support, and providing a higher concentration of oxygen. Scientists and clinicians are still refining this treatment, as lung damage can also result from ventilating the lungs at too high a pressure or from providing too high an oxygen concentration.
Intrapleural Space
The left and right lungs each sit within their own cavity within the thorax. Each cavity is bounded by the ribcage, diaphragm, and pericardium (the sac surrounding the heart). These boundaries are lined by a pleural membrane, and another pleural membrane envelopes the lung (Figure 14.23). The pleural membranes touch and slide past each other as the lung expands and shrinks with every breath. To minimize friction as the membranes slide, there is a small amount of serous fluid between them that acts as a lubricant (imagine holding a plastic zip-top bag with a little bit of water inside [and no air] and sliding the walls of the bag past each other). The space between the membranes is called the pleural space, and it is considered a “potential space” because it is extremely small in healthy people. The pleural spaces (left and right) are crucial to our ability to breathe. If an injury causes a tear in one of the pleural membranes, the potential space will become an actual space, and the lung will collapse.
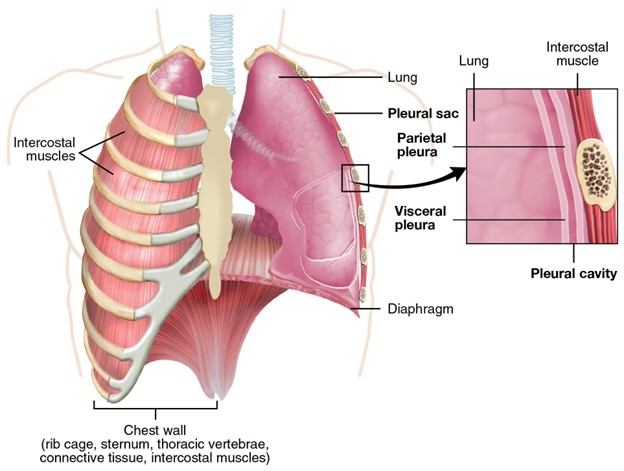
Figure 14.23—Anatomy of human lungs and pleural cavity. Each lung is located within a separate pleural cavity. The outside of each lung is covered in a visceral pleural membrane (i.e., pleura), and the margins of the pleural cavity (the rib cage, diaphragm, and pericardium) are lined by a parietal pleura. Between the visceral and parietal pleurae is the pleural cavity, which contains a small amount of pleural fluid.
Pneumothorax and hemothorax occur when air or blood (respectively) enters the pleural space. These occur when either the chest wall or the wall of the lung is damaged. This can be caused by blunt trauma (e.g., fractured rib, knife wound, gunshot wound), by lung diseases (e.g., COPD [chronic obstructive pulmonary disease], cystic fibrosis, tuberculosis), by connective tissue disorders (e.g., Marfan’s syndrome), or by certain medical procedures. Because each lung is surrounded by a separate pleural space, only the lung on the affected side will collapse.
In certain cases, called tension pneumothorax, the damaged pleural membrane acts as a one-way valve. Air enters the pleural space as the person breathes in but cannot leave the pleural space as the person breathes out. This causes air to accumulate in the pleural space, which ultimately puts pressure on the other lung and the heart, pushing them aside within the thoracic cavity and making it more difficult for the heart to pump. This is treated by needle thoracostomy—inserting a needle into the pleural space so that air is no longer trapped there.
Box 14.5—Reading Human Chest Radiographs
Imaging the lungs enables physicians to diagnose abnormal, injured, or diseased conditions. Radiography (X-ray imaging) is well suited for visualizing the lungs because X-rays produce contrast when adjacent structures have different densities (or, more accurately, different X-ray absorption coefficients), and the lungs are radio-lucent structures surrounded by radio-opaque structures. X-rays easily pass through the lungs but are absorbed into the soft tissue surrounding them, making it easy to see the margins of the lungs, fluid in the lungs, diseased or scarred lung tissue, or other indications of injury or poor health. In the images provided here, radio-lucent structures (such as the lungs) appear dark, and radio-dense structures (such as bones and muscles) appear bright.
In order to recognize when something is wrong, physicians must be familiar with how healthy lungs appear in chest X-rays. The first image is a chest X-ray of a person with relatively healthy lungs (however, note that they have cardiomegaly [i.e., an enlarged heart], and there are artifacts in the image related to their treatment, specifically four EKG [electrocardiogram] leads and circular artifacts along the midline; Figure 14.24). Before dragging the slider over to look at the second image, try to identify the location of the anatomical features listed on the left and right of the image. When you are ready, look at the second image, where these features are labeled.
(Note: Hemidiaphragms are the left and right halves of the diaphragm. Costodiaphragmatic recesses are the potential spaces where the costal and diaphragmatic pleura meet and where fluid is likely to pool.)
Next let us look at a chest X-ray of a person with a diseased lung (Figure 14.25). Examine the image, consider the position of the left lung, consider the radio density of the material in the left pleural cavity, and then describe what condition you think this person has. When you are ready, drag the slider over to look at the following image and see the labels.
Lung Capacities
When a healthy adult breathes in as deeply as they can, their lungs are filled with 6 L of air, on average (total lung capacity, TLC). And when a healthy adult exhales as deeply as they can, their lungs contain an average of 1.2 L of air (residual volume, RV). Yet the average amount of air that is exhaled and inhaled with each breath at rest is only 450 mL (tidal volume, TV). These terms (TLC, RV, TV) and many others have been created to refer to the volume of air in the lungs at different time points during respiration (Figure 14.26). By quantifying typical values for these volumes in healthy populations, clinicians have determined what values are abnormal and indicate impaired respiratory performance.
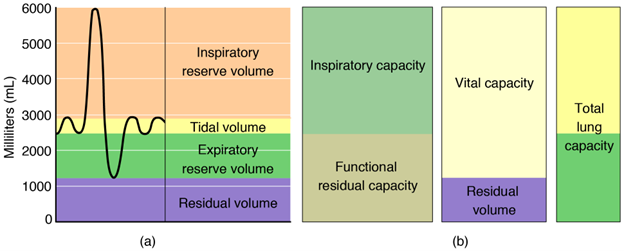
Figure 14.26—Graph of human lung volumes and capacities. Volume is plotted in milliliters. (A) Respiratory volumes during breathing at rest and during maximal ventilation. (B) Lung capacities that combine to equate to total lung capacity. Tidal volume: the volume of air ventilated during a typical breath at rest. Inspiratory capacity: maximum amount of air that can be inhaled at the end of a normal exhalation at rest. Inspiratory reserve volume: inspiratory capacity minus tidal volume. Expiratory reserve volume: the maximum amount of air that can be exhaled, excluding tidal volume. Residual volume: the volume of air that remains in the lungs at maximal exhalation. Functional residual capacity: the amount of air that remains in the lungs after a typical exhalation at rest. Vital capacity: the maximum amount of air that can be exhaled after taking a maximal inhalation.
One common test that clinicians use to assess respiratory performance is spirometry. While breathing through a spirometer, a patient is asked to inhale as deeply as possible and then exhale as forcefully as possible (forced vital capacity, FVC). From this, the volume of air that was forcefully exhaled during the first second of exhalation (FEV1) can be measured. These measurements can help diagnose a variety of diseases, such as COPD or asthma. For example, a healthy patient expels 90% of FVC during the first second of forced exhalation, but a patient with asthma may expel only 50% of FVC during the first second.
Airway Protection
Our respiratory system needs to protect itself from material, both big and small, that enters the airways. When denser material, such as liquid or a piece of food, slips past the epiglottis and enters the space above the vestibular and vocal folds, it triggers the vestibular folds to quickly close. This prevents the material from entering the trachea, but it also blocks the exhalation and inhalation of air. Sometimes it is actually the vestibular folds, not the ingested material, that block the airways and create the sensation of choking. The Heimlich maneuver remedies this by creating sufficient pressure in the airways to push the vestibular folds open and eject the food or liquid. Aspiration occurs when material passes the vestibular folds and enters the lungs. The left bronchus passes above the heart, so it is more horizontal than the right bronchus. As a result, aspirated bodies are more likely to enter the right bronchus.
In addition to larger or denser material, our respiratory system also must protect itself from airborne particles and pathogens. Mucus is produced by the epithelium lining the airways and traps particulates. Cilia sweep the mucus up toward the larynx, where it exits the airways and is swallowed. However, problems occur when the respiratory system overreacts to the presence of particulate matter. This occurs in people who suffer from asthma, resulting in inflammation, overproduction of mucus in the airways, and bronchospasm (contraction of the muscles that line the bronchi, causing the airways to narrow and making it difficult to breathe). Problems also occur when the respiratory system is outmatched by more particulate matter (including cigarette smoke) than it can clear away. This results in occupational or environmental lung diseases, such as emphysema, chronic bronchitis, silicosis, and black lung disease. Last, the respiratory system must rely on the immune system to protect itself from pathogens, including bacteria, viruses, and fungi.
14.8 Summary
The body’s metabolic processes necessitate intaking oxygen and expelling carbon dioxide. Beyond very small body sizes, the physics of gas exchange becomes unfavorable quickly. To overcome these challenges, vertebrates have evolved several different mechanisms for optimizing gas exchange. There are two broad sets of respiratory organs: gills for gas exchange with water and lungs for gas exchange with air. Principally, both strategies increase their capacity and efficiency through three methods: (1) increasing surface area available for gas exchange, (2) maximizing the efficiency of flow through the respiratory system, and (3) maximizing the efficiency of the interaction of the respiratory system with the cardiovascular system. How these methods are employed varies across vertebrates, with evolutionary history and ecology playing a strong role.
Application Questions
- Gills and lungs fulfill essentially the same job: extracting oxygen from the environment. What are the functional limitations that prevent a water-filled lung from working underwater?
- When in development do most animals begin to have oxygen requirements?
- What are some examples of respiratory evolution either constraining or being constrained by other organ systems?
- How would life differ if breathing required skeletal muscles actively controlled by the brain and could not occur passively?
- Why is it important for the ventilation rate to match the physiological need for gas exchange? What happens if the ventilation rate is too fast or too slow?
- Countercurrent exchange is one way that gills continue to exchange gases, even in low-oxygen water. This form of exchange requires a specific orientation of fluid flows, and we actually see remarkably similar configurations across the tree of life, from penguin feet, to gas glands in many fish, to the nephrons in your own kidney. Why do you think these countercurrent exchange structures have independently evolved so many times?
14.9 Further Reading
- Graham, Jeffrey B. 1997. Air-Breathing Fishes—Evolution, Diversity and Adaptation. San Diego, CA: Academic Press.
- Perry, Steven F., Markus Lambertz, and Anke Schmitz. 2019. Respiratory Biology of Animals: Evolutionary and Functional Morphology. London, England: Oxford University Press.
- Maina, John N. 2002. Biological Systems in Vertebrates, Vol. 1: Functional Morphology of the Vertebrate Respiratory Systems. Biological Systems in Vertebrates. Enfield, CT: Science.
- Weibel, Ewald R. 1984. The Pathway for Oxygen: Structure and Function in the Mammalian Respiratory System. Harvard University Press.
14.10 References
- Belkin, Daniel A. 1968. “Aquatic respiration and underwater survival of two freshwater turtle species.” Respiration Physiology 4 (1): 1–14. https://doi.org/10.1016/0034-5687(68)90002-9.
- Brainerd, E. L. 1999. “New perspectives on the evolution of lung ventilation mechanisms in vertebrates.” Experimental Biology Online 4 (2): 1–28. https://doi.org/10.1007/s00898-999-0002-1.
- Brainerd, Elizabeth L., Jeremy S. Ditelberg, and Dennis M. Bramble. 1993. “Lung ventilation in salamanders and the evolution of vertebrate air-breathing mechanisms.” Biological Journal of the Linnean Society 49 (2): 163–183. https://doi.org/10.1111/j.1095-8312.1993.tb00896.x.
- Brainerd, Elizabeth L., and Lara A. Ferry-Graham. 2005. “Mechanics of respiratory pumps.” In Fish Physiology, 23:1–28. Fish Biomechanics. Academic Press. https://doi.org/10.1016/S1546-5098(05)23001-7.
- Brainerd, Elizabeth L., and Tomasz Owerkowicz. 2006. “Functional morphology and evolution of aspiration breathing in tetrapods.” Respiratory Physiology & Neurobiology, Frontiers in Comparative Physiology II: Respiratory Rhythm, Pattern and Responses to Environmental Change, 154 (1): 73–88. https://doi.org/10.1016/j.resp.2006.06.003.
- Bretz, William L., and Knut Schmidt-Nielsen. 1971. “Bird respiration: Flow patterns in the duck lung.” Journal of Experimental Biology 54 (1): 103–118. https://doi.org/10.1242/jeb.54.1.103.
- Brocklehurst, Robert J., Emma R. Schachner, Jonathan R. Codd, and William I. Sellers. 2020. “Respiratory evolution in archosaurs.” Philosophical Transactions of the Royal Society B 375 (1793): 20190140. https://doi.org/10.1098/rstb.2019.0140.
- Capano, John G., Scott M. Boback, Hannah I. Weller, Robert L. Cieri, Charles F. Zwemer, and Elizabeth L. Brainerd. 2022. “Modular lung ventilation in boa constrictor.” Journal of Experimental Biology 225 (6): jeb243119. https://doi.org/10.1242/jeb.243119.
- Carrier, David R. 1987. “The evolution of locomotor stamina in tetrapods: Circumventing a mechanical constraint.” Paleobiology 13 (3): 326–341. https://doi.org/10.1017/S0094837300008903.
- Cass, Amanda N., Marc D. Servetnick, and Amy R. McCune. 2013. “Expression of a lung developmental cassette in the adult and developing zebrafish swimbladder.” Evolution and Development 15 (2): 119–132. https://doi.org/10.1111/ede.12022.
- Cieri, Robert L., Brent A. Craven, Emma R. Schachner, and C. G. Farmer. 2014. “New insight into the evolution of the vertebrate respiratory system and the discovery of unidirectional airflow in iguana lungs.” Proceedings of the National Academy of Sciences U.S.A. 111 (48): 17218–17223.
- Cieri, Robert L., and Colleen G. Farmer. 2020. “Computational fluid dynamics reveals a unique net unidirectional pattern of pulmonary airflow in the savannah monitor lizard (Varanus exanthematicus).” The Anatomical Record 303 (7): 1768–1791. https://doi.org/10.1002/ar.24293.
- Cieri, Robert L., Samuel T. Hatch, John G. Capano, and Elizabeth L. Brainerd. 2020. “Locomotor rib kinematics in two species of lizards and a new hypothesis for the evolution of aspiration breathing in amniotes.” Scientific Reports 10 (1): 7739. https://doi.org/10.1038/s41598-020-64140-y.
- Claessens, Leon P. A. M. 2009. “The skeletal kinematics of lung ventilation in three basal bird taxa (Emu, Tinamou, and Guinea Fowl).” Journal of Experimental Zoology A: Ecological Genetics and Physiology 311A (8): 586–599. https://doi.org/10.1002/jez.501.
- Cruz, André Luis da, Bruno Vilela, and Wilfried Klein. 2023. “Morphological and physiological traits of the respiratory system in Iguana Iguana and other non-avian reptiles.” Zoology 157 (April): 126079. https://doi.org/10.1016/j.zool.2023.126079.
- Cupello, Camila, Tatsuya Hirasawa, Norifumi Tatsumi, Yoshitaka Yabumoto, Pierre Gueriau, Sumio Isogai, Ryoko Matsumoto, et al. 2022. “Lung evolution in vertebrates and the water-to-land transition.” eLife 11 (July): e77156. https://doi.org/10.7554/eLife.77156.
- Daniels, Christopher B., Sandra Orgeig, and Allan W. Smits. 1995. “The evolution of the vertebrate pulmonary surfactant system.” Physiological Zoology 68 (4): 539–566. https://doi.org/10.1086/physzool.68.4.30166344.
- De Padova, Jordan, Nigel K. Anderson, Roland Halbauer, Doris Preininger, and Matthew J. Fuxjager. 2024. “Acute hypoxia exposure rapidly triggers behavioral changes linked to cutaneous gas exchange in Lake Titicaca frogs.” Behavioural Processes 219 (June): 105047. https://doi.org/10.1016/j.beproc.2024.105047.
- Dotterweich, Heinz. 1930. “Versuche über den weg der atemluft in der vogellunge.” Zeitschrift für Vergleichende Physiologie 11 (2): 271–284. https://doi.org/10.1007/BF00338870.
- Duncker, Hans-Rainer. 2004. “Vertebrate lungs: Structure, topography and mechanics: A comparative perspective of the progressive integration of respiratory system, locomotor apparatus and ontogenetic development.” Respiratory Physiology & Neurobiology, Frontiers in Comparative Respiratory Physiology 1: Adaptations in Respiratory Gas Transport 144 (2): 111–124. https://doi.org/10.1016/j.resp.2004.07.020.
- Farmer, C. G., and D. C. Jackson. 1998. “Air-breathing during activity in the fishes Amia calva and Lepisosteus oculatus.” Journal of Experimental Biology 201 (7): 943–948. https://doi.org/10.1242/jeb.201.7.943.
- Farmer, Colleen. 1997. “Did lungs and the intracardiac shunt evolve to oxygenate the heart in vertebrates?” Paleobiology 23 (3): 358–372. https://doi.org/10.1017/S0094837300019734.
- Farmer, Colleen G. 2015. “Unidirectional flow in lizard lungs: A paradigm shift in our understanding of lung evolution in Diapsida.” Zoology 118 (5): 299–301. https://doi.org/10.1016/j.zool.2015.06.001.
- Farmer, Colleen G., and Kent Sanders. 2010. “Unidirectional airflow in the lungs of alligators.” Science 327 (5963): 338–340. https://doi.org/10.1126/science.1180219.
- Feder, Martin E., and Warren W. Burggren. 1985. “Skin breathing in vertebrates.” Scientific American 253 (5): 126–143.
- FitzGibbon, Sean, and Craig Franklin. 2011. “The importance of the cloacal bursae as the primary site of aquatic respiration in the freshwater turtle, Elseya albagula.” Australian Zoologist 35 (2): 276–282. https://doi.org/10.7882/AZ.2010.016.
- Gaunt, Abbot S., and Carl Gans. 1969. “Mechanics of respiration in the snapping turtle, Chelydra serpentina (Linné).” Journal of Morphology 128 (2): 195–227. https://doi.org/10.1002/jmor.1051280205.
- Gee, John H., and Jeffrey B. Graham. 1978. “Respiratory and hydrostatic functions of the intestine of the catfishes Hoplosternum thoracatum and Brochis splendens (Callichthyidae).” Journal of Experimental Biology 74 (1): 1–16. https://doi.org/10.1242/jeb.74.1.1a.
- Green, Bridget S., and Mark I. McCormick. 2005. “O2 Replenishment to fish nests: Males adjust brood care to ambient conditions and brood development.” Behavioral Ecology 16 (2): 389–397. https://doi.org/10.1093/beheco/ari007.
- Hazelhoff, Engel Hendrik. 1943. “Bouw en functie van de vogellong.” Publications of Famous Biologists-a Collection of Prints.
- Hedrick, Michael S., and Stephen L. Katz. 2015. “Control of breathing in primitive fishes.” In Phylogeny, Anatomy and Physiology of Ancient Fishes. CRC Press.
- Hislop, Alison A. 2002. “Airway and blood vessel interaction during lung development.” Journal of Anatomy 201 (4): 325–334. https://doi.org/10.1046/j.1469-7580.2002.00097.x.
- Hutchison, Victor H., Howard B. Haines, and Gustav Engbretson. 1976. “Aquatic life at high altitude: Respiratory adaptations in the Lake Titicaca frog, Telmatobius culeus.” Respiration Physiology 27 (1): 115–129. https://doi.org/10.1016/0034-5687(76)90022-0.
- Janis, C. M., and J. C. Keller. 2001. “Modes of ventilation in early tetrapods: Costal aspiration as a key feature of amniotes.” Acta Palaeontologica Polonica 46 (2). http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-article-1106db6a-71af-4eee-8f7e-2b57bcf4bb4c.
- Johansen, K. 1971. “Comparative physiology: Gas exchange and circulation in fishes.” Annual Review of Physiology 33 (Volume 33, 1971): 569–612. https://doi.org/10.1146/annurev.ph.33.030171.003033.
- Kaczmarek, Elska B., and Elizabeth L. Brainerd. 2024. “Buoyancy control and air breathing in royal knifefish (Chitala blanci) and a new hypothesis for the early evolution of vertebrate air-breathing behaviors.” The Anatomical Record, May 7, 2024, 1–15. https://doi.org/10.1002/ar.25460.
- Kaczmarek, Elska B, Samantha M Gartner, Mark W Westneat, and Elizabeth L Brainerd. 2022. “Air breathing and suction feeding kinematics in the West African lungfish, Protopterus annectens.” Integrative and Comparative Biology 62 (4): 865–877. https://doi.org/10.1093/icb/icac109.
- Kerney, Ryan. 2011. “Symbioses between salamander embryos and green algae.” Symbiosis 54 (3): 107–117. https://doi.org/10.1007/s13199-011-0134-2.
- Koos, Brian J., and Arezoo Rajaee. 2014. “Fetal breathing movements and changes at birth.” In Advances in Fetal and Neonatal Physiology, edited by Lubo Zhang and Charles A. Ducsay, 89–101. New York, NY: Springer. https://doi.org/10.1007/978-1-4939-1031-1_8.
- Liem, Karel F. 1988. “Form and function of lungs: The evolution of air-breathing mechanisms.” American Zoologist 28 (2): 739–759. https://doi.org/10.1093/icb/28.2.739.
- Longo, Sarah, Mark Riccio, and Amy R McCune. 2013. “Homology of lungs and gas bladders: Insights from arterial vasculature.” Journal of Morphology 274 (6): 687–703. https://doi.org/10.1002/jmor.20128.
- Meir, Jessica U, Julia M York, Bev A Chua, Wilhelmina Jardine, Lucy A Hawkes, and William K Milsom. 2019. “Reduced metabolism supports hypoxic flight in the high-flying bar-headed goose (Anser indicus).” Edited by Harry C Dietz, Iain D Couzin, and Jon Harrison. eLife 8 (September): e44986. https://doi.org/10.7554/eLife.44986.
- Ochs, Matthias, Jens R. Nyengaard, Anja Jung, Lars Knudsen, Marion Voigt, Thorsten Wahlers, Joachim Richter, and Hans Jørgen G. Gundersen. 2004. “The number of alveoli in the human lung.” American Journal of Respiratory and Critical Care Medicine 169 (1): 120–124. https://doi.org/10.1164/rccm.200308-1107OC.
- Owerkowicz, Tomasz, Colleen G. Farmer, James W. Hicks, and Elizabeth L. Brainerd. 1999. “Contribution of gular pumping to lung ventilation in monitor lizards.” Science 284 (5420): 1661–1663. https://doi.org/10.1126/science.284.5420.1661.
- Palmer, Michael A., Bryan A. Nerger, Katharine Goodwin, Anvitha Sudhakar, Sandra B. Lemke, Pavithran T. Ravindran, Jared E. Toettcher, Andrej Košmrlj, and Celeste M. Nelson. 2021. “Stress ball morphogenesis: How the lizard builds its lung.” Science Advances 7 (52): eabk0161. https://doi.org/10.1126/sciadv.abk0161.
- Perry, Steven F, Richard J. A Wilson, Christian Straus, Michael B Harris, and John E Remmers. 2001. “Which came first, the lung or the breath?” Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology, Surfactant, Lungs and the Evolution of Air Breathing—Tribute to R. E. Pattle, 129 (1): 37–47. https://doi.org/10.1016/S1095-6433(01)00304-X.
- Phillips, Jackson R, Gary K Nicolau, Shane S Ngwenya, Emily A Jackson, and Molly C Womack. 2024. “Habitat and respiratory strategy effects on hypoxia performance in anuran tadpoles.” Integrative and Comparative Biology 64 (2): 336–353. https://doi.org/10.1093/icb/icae017.
- Piiper, J., and P. Scheid. 1982. “Models for a comparative functional analysis of gas exchange organs in vertebrates.” Journal of Applied Physiology 53 (6): 1321–1329. https://doi.org/10.1152/jappl.1982.53.6.1321.
- Powell, Frank L., and Susan R. Hopkins. 2004. “Comparative physiology of lung complexity: Implications for gas exchange.” Physiology 19 (2): 55–60. https://doi.org/10.1152/nips.01469.2003.
- Roth, Gerhard, and David B. Wake. 1985. “Trends in the functional morphology and sensorimotor control of feeding behavior in salamanders: An example of the role of internal dynamics in evolution.” Acta Biotheoretica 34 (2): 175–191. https://doi.org/10.1007/BF00046783.
- Sanchez-Esteban, Juan, Lawrence A. Cicchiello, Yulian Wang, Shu-Whei Tsai, Lakisha K. Williams, John S. Torday, and Lewis P. Rubin. 2001. “Mechanical stretch promotes alveolar epithelial type II cell differentiation.” Journal of Applied Physiology 91 (2): 589–595. https://doi.org/10.1152/jappl.2001.91.2.589.
- Schachner, Emma R., John R. Hutchinson, and C. G. Farmer. 2013. “Pulmonary anatomy in the Nile crocodile and the evolution of unidirectional airflow in archosauria.” PeerJ 1 (March): e60. https://doi.org/10.7717/peerj.60.
- Schachner, Emma R., Andrew J. Moore, Aracely Martinez, Raul E. Diaz Jr., M. Scott Echols, Jessie Atterholt, Roger W. P. Kissane, Brandon P. Hedrick, and Karl T. Bates. 2024. “The respiratory system influences flight mechanics in soaring birds.” Nature 630 (8017): 671–676. https://doi.org/10.1038/s41586-024-07485-y.
- Scheid, Peter, and Johannes Piiper. 1972. “Cross-current gas exchange in avian lungs: Effects of reversed parabronchial air flow in ducks.” Respiration Physiology 16 (3): 304–312. https://doi.org/10.1016/0034-5687(72)90060-6.
- Schmidt-Nielsen, Knut. 1971. “How birds breathe.” Scientific American 225 (6): 72–79.
- Schwenk, Kurt, and Jackson R. Phillips. 2020. “Circumventing surface tension: Tadpoles suck bubbles to breathe air.” Proceedings of the Royal Society B: Biological Sciences 287 (1921): 20192704. https://doi.org/10.1098/rspb.2019.2704.
- Souza, Ray Brasil Bueno de, Vanessa Maria Gomes Bonfim, Vitor Passos Rios, and Wilfried Klein. 2021. “Allometric relations of respiratory variables in amniota: Effects of phylogeny, form, and function.” Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology 252 (February): 110845. https://doi.org/10.1016/j.cbpa.2020.110845.
- Speck, Dexter F., Michael S. Dekin, W. Robert Revelette, and Donald A. Frazier. 2014. Respiratory Control: Central and Peripheral Mechanisms. University Press of Kentucky.
- Starck, J. Matthias, James R. Stewart, and Daniel G. Blackburn. 2021. “Phylogeny and evolutionary history of the amniote egg.” Journal of Morphology 282 (7): 1080–1122. https://doi.org/10.1002/jmor.21380.
- Steen, Johan B. 1970. “10 The swim bladder as a hydrostatic organ.” In Fish Physiology, edited by W. S. Hoar and D. J. Randall, 4:413–443. The Nervous System, Circulation, and Respiration. Academic Press. https://doi.org/10.1016/S1546-5098(08)60135-1.
- Uriona, T J, M Lyon, and C G Farmer. 2019. “Lithophagy prolongs voluntary dives in American alligators (Alligator mississippiensis).” Integrative Organismal Biology 1 (1): oby008. https://doi.org/10.1093/iob/oby008.
- Vos, H. J. 1934. “Über den weg der atemluft in der entenlunge.” Zeitschrift für Vergleichende Physiologie 21 (4): 552–578. https://doi.org/10.1007/BF00340621.
- Wake, David B., and Stephen M. Deban. 2002. “Terrestrial feeding in salamanders.” In Feeding: Form, Function and Evolution in Tetrapod Vertebrates, edited by Kurt Schwenk, 95–116. San Diego, CA: Academic Press.
- Wang, Nai-San. 1998. “Anatomy of the pleura.” Clinics in Chest Medicine 19 (2): 229–240. https://doi.org/10.1016/S0272-5231(05)70074-5.
- Warburton, David, Ahmed El-Hashash, Gianni Carraro, Caterina Tiozzo, Frederic Sala, Orquidea Rogers, Stijn De Langhe, et al. 2010. “Lung organogenesis.” In Current Topics in Developmental Biology, edited by Peter Koopman, 90: 73–158. Organogenesis in Development. Academic Press. https://doi.org/10.1016/S0070-2153(10)90003-3.
- Warren, Jamie B., and JoDee M. Anderson. 2009. “Core concepts: Respiratory distress syndrome.” NeoReviews 10 (7): e351–e361. https://doi.org/10.1542/neo.10-7-e351.

