3 Vertebrate Origins
Lisa B. Whitenack
Focus Questions—to Guide Your Reading of This Chapter
- What are some of the defining characteristics of vertebrates? Gnathostomes? Eugnathostomes?
- What familiar vertebrates fall into each taxonomic group of vertebrates?
3.1 Introduction—What Is a Vertebrate, Anyway?
In Chapter 2, we introduced you to deuterostomes and, more specifically, the chordates. We also introduced you to the vertebrates in general, including what early vertebrates may have looked like. Let’s dive deeper into some features that vertebrates share, and we’ll add some new ones to your list.
Neural crest cells have been regarded as one of the most important unique vertebrate features. The cells develop along either side of the neural plate, which makes up the embryonic nerve cord (neural tube) and embryonic brain (Figure 3.1). You will read more about these and what they become in Chapter 4, but they have two important properties. The first is that they are migratory. Across vertebrates, we see these cells develop at the neural plate from anterior to posterior, and then they distribute themselves around the body. Once they arrive at their destination, we see their second important property: They are multipotent. That means that they can become many different things. They can make the structural tissues in your head (bone, cartilage, dentine in your teeth), nervous system cells, or pigment cells, depending on which genes are expressed and when. Altogether, neural crest cells are responsible for many vertebrate features.

Figure 3.1—Neural crest cell formation during the process of neurulation. Note that the neural tube develops into the dorsal nerve cord.
Let’s now imagine a vertebrate—it might be you, your cat, or your goldfish. Regardless, whichever vertebrate you’ve imagined has a head. If we had to define what a head is, we would say something like “it’s the part of the body where the mouth, brain, skull, and sensory organs are located.” Note that we didn’t say there was a jaw or teeth—not all vertebrates have these (more on that later). It’s also worth noting that many invertebrates have heads (albeit without skulls) too—insects are great examples.
Considering the vertebrate head leads us to more vertebrate features. The first was mentioned in the previous chapter, cranial ectodermal placodes, also called neurogenic placodes (Figure 3.2). These embryonic features are a thickening of tissue at various places in the head region. Based on interactions between cells and tissues, placodes can differentiate into a number of different structures depending on their location. These include the lens of the vertebrate eye, portions of various cranial nerves (nerves that come directly off the brain), structures related to olfaction (sense of smell), and more. You will read more about these placodes in Chapter 4 and about some of their derivatives in the chapters about the nervous and sensory system (Chapters 18, 19, and 20).

Figure 3.2—The olfactory (yellow), lens (blue), trigeminal (green), otic (purple), and epibranchial (orange) cranial ectodermal (neurogenic) placodes locate to characteristic positions, here shown in a side view of a three-day-old chick embryo. You will learn much more about these names and structures in later chapters.
Speaking of nervous systems, vertebrates are also known for their fairly complex brains. If the vertebrate you thought of earlier was a goldfish, you may not categorize its brain as “complex.” However, it’s all relative—if we compare vertebrate brains to those of other chordates, for example, even that goldfish brain is pretty complex. Vertebrate brains are divided into three regions: a forebrain, a midbrain, and a hindbrain (Figure 3.3). These are very distinct regions that have fairly distinct functions, even in goldfish. Cephalochordates, on the other hand, do not have any obvious divisions at all. They have a slightly larger mass of neural tissue in their head region called the cerebral vesicle. There is some difference in gene expression as you move anterior to posterior in the cerebral vesicle, but that’s it. Urochordates have no brain at all. Neural crest cells are not responsible for making any vertebrate brain tissue itself. However, they do help make some of the cranial nerves coming off the brain. You’ll learn more about these in Chapter 19.

Figure 3.3—Divisions of the embryonic vertebrate brain during different periods of development.
Vertebrates protect their brains with a skull, also called the cranium. This is typically made up of either cartilage or bone, which means that the skull is a feature that is derived from those very important neural crest cells. Skulls are fascinating parts of the body that can tell you about sensory systems, the brain itself, posture, feeding mode, and much more. In fact, we’ve devoted an entire chapter just to the skull (Chapter 8).
If we could summarize everything we’ve just discussed, we could say that vertebrates are defined by their heads. Indeed, as scientists began to understand the importance of neural crest cells and the neurogenic placodes, Carl Gans and Glenn Northcutt proposed the “New Head Hypothesis” in 1983. The hypothesis contains several different strands that concern the origin of unique vertebrate tissues, homology, and much more. The general idea is this:
- Neural crest cells and placodes, their resulting features, and some innovations in the pharynx all resulted in a change from filter feeding to active predation.
- This switch in feeding mode opened up several new niches and allowed vertebrates to flourish.
The various strands of the New Head Hypothesis have undergone extensive testing. The importance of neural crest cells and neurogenic placodes to vertebrate evolution has held true. However, as technology continues to advance and allow new ways to test these ideas, some have not been supported. This includes the trend of more active predation through early vertebrate evolution.
Let’s move posteriorly along the vertebrate body. As we leave the head, we encounter skeletal elements called vertebrae (sing. = vertebra), which are informally called a backbone and are part of the axial skeleton (which also includes the skull, ribs, and parts of the pharynx). Each vertebra is composed of either bone, cartilage, or other connective tissue, depending on which vertebrate you’re examining. These supportive structures usually surround the notochord, and in many vertebrates, the notochord is incorporated into the vertebra or its nearby structures to varying degrees. Most vertebrae are composed of two distinct structures, a centrum (the body of the vertebra) and vertebral arches (located dorsal and ventral to the centrum), although several vertebrate groups only have one or the other (Figure 3.4). Vertebral morphology varies greatly across taxa and regionally within a species, making it useful for understanding locomotion, taxonomy (classification), and the transition of vertebrates from aquatic to terrestrial environments. Once again, we have an entire chapter devoted to the noncranial parts of the axial skeleton (Chapter 9).

Figure 3.4—Mammalian thoracic vertebra. Note that this vertebra only has a vertebral arch dorsal to the centrum.
For our final vertebrate feature, we are going to move from structures to genes. Hox genes, which we introduced in the previous chapter, control anterior-posterior body patterning in bilateral animals (both invertebrates and vertebrates). In the vertebrates, they are also associated with the cranial neural crest cells and appendages, among other things. How did this happen? If we go back to the invertebrates, the Hox genes are located within a single, compact cluster. However, in vertebrates, these clusters have gone through several rounds of duplication (Figure 3.5). As a result, vertebrates have somewhere between three and eight clusters of Hox genes, depending on which group of vertebrates you’re considering. Once important genes are duplicated, constraints on some of those copies can be lifted, allowing natural selection to act on one copy without detrimental effects. What do the nonvertebrate chordates have? Amphioxus, one of our living cephalochordates, has just one Hox cluster. Interestingly, extant urochordates that have been studied have no Hox clusters, although they do have Hox genes. We have to once again remember that our living nonvertebrate chordates diverged from the vertebrates over 500 million years ago, and their genome has had a lot of time to change. It is likely that urochordates probably had at least a single Hox cluster at some point and have lost it over time.

Figure 3.5—Despite rearrangements, inversions, and duplications, all bilaterally symmetrical animals contain recognizably comparable sets of Hox genes. Mammals, such as mice and humans, have four clusters of Hox genes (labeled HOXA–HOXD) that are expressed along the anterior-posterior axis.
3.2 Let’s Meet the Vertebrates
So far, we’ve determined that the vertebrates have a head and vertebrae, as well as embryonic and genetic similarities. Which living taxa have these? You do, as do other mammals. So do birds, reptiles, amphibians, and fishes of all kinds. We also have a lot of extinct vertebrates to account for across their 500+ million years of existence. All these vertebrate groups have their own suite of characteristics that define them, and we use these characteristics to figure out vertebrate evolutionary relationships (Box 3.1). As we dive into the different groups of vertebrates, keep in mind that the evolutionary relationships we discuss here may change in the future as scientists continue to study the various vertebrate groups. Similarly, you may find phylogenetic trees with other branching patterns if you look at other sources of information, depending on when those trees were generated and what kind of data they used to build them.
Box 3.1—Phylogenetic Trees Are Hypotheses
When we introduced you to phylogenetic trees in the last chapter, we were concerned with how to read them. However, it’s also important to understand how those trees are generated in the first place—Why is one taxon linked more closely to another taxon, compared to a third? Why are the nodes placed the way they are? Why do phylogenetic trees with the exact same taxa differ in branching pattern depending on what source you’re looking at?
Two general components are important in making phylogenetic trees—the data that you input and the evolutionary model that you chose to apply those data to. The choices that scientists make for each of those components come with particular assumptions, strengths, and weaknesses. Not all data types or evolutionary models are appropriate for all evolutionary questions. In other words, scientists need to choose the most applicable data and models for the evolutionary questions they are seeking to answer.
The data that are collected to generate these trees can be either morphological or molecular. Morphological data are essentially anything you can measure on the organism, whether that is a feature (e.g., presence/absence or counting something), a shape (e.g., length, width, oval), or physiological characteristics (e.g., the ability to break down lactose). When incorporating fossil data into phylogenies, you are limited to morphological characteristics. One also must consider if the characteristics they want to measure are actually informative for the evolutionary question or not. For example, if one were interested in the evolutionary relationships of sharks, the presence or absence of an eye is not informative because all sharks have eyes!
Molecular data generally concern the sequence of genes in some way. We can use DNA or RNA, genetic information from the nucleus or mitochondria, genetic or amino acid sequences, whole or partial gene sequences, and more. Which of these things we choose matters just as much as which morphological characters we choose. For example, mutation rates vary across genes, between mitochondrial and nuclear genes, and even within a single RNA strand. If our research question concerns evolution in a very short time frame, we would want to focus on a gene that has a high mutation rate; otherwise, we may not see any differences in sequence. Similarly, if our research question concerns a very long time frame (such as all of vertebrate evolution), we may want a gene that has a very slow mutation rate so that any differences due to evolutionary relationships are not swamped by noise from many mutations over 500 million years.
Once we have the data, we have to apply evolutionary models to it. For morphological data, this means making decisions about which traits are more ancestral than others and coding them accordingly (or making a decision to not code the data this way). We then have to decide how we want the computer to apply evolutionary processes to these data—Do we allow evolutionary reversals? What do we do when branching patterns are equally likely? Do we apply statistical analyses to help figure that out? Do we want random evolution or more directed evolutionary processes? We have similar decisions to make for molecular evolution models, but we have to add in other considerations, such as assumptions about mutation rates and what kinds of mutations are more likely than others.
There are entire graduate school courses on the data and models that we use to make these trees, and our goal is only to give you a taste of this here. The point we want you to take from this is that there are a lot of human-generated decisions that go into making phylogenetic trees. Even if we stick with molecular trees, changing which gene you use can result in a different branching pattern. Thus, phylogenetic trees are evolutionary hypotheses, not definitive answers. When we see the same patterns coming up with multiple types of data and models, we have strong evidence that the relationships we see in the trees—those hypotheses—are the most likely scenario. The phylogenetic trees in this book, and the hypotheses they represent, fall into that category as of the writing of this textbook in 2025.
We can divide the Vertebrata into two broad groups, Cyclostomata (hagfishes and lampreys) and Gnathostomata (everything else; Figure 3.6).

Figure 3.6—Phylogenetic tree of the Vertebrata. Dotted lines indicate that the placement of the branch is uncertain. The dagger (†) indicates that all members of that taxonomic group are extinct.
Cyclostomata
The cyclostomes include extant hagfishes (Mixinoidea, Figure 3.7) and lampreys (Petromyzontidae, Figure 3.8) and their extinct relatives. The word Cyclostomata translates to “circle mouth,” which aptly describes the most prominent feature of lampreys. Hagfishes and lampreys have other morphological features in common as well. Both are jawless and have teeth made of keratin (a protein that your nails and hair are also made of). Both groups have a single nostril, gill pouches, and evolutionary precursors to vertebrae called arcualia. Both are long and don’t have scales. Beyond that, the morphological similarities are few. Most of the support for placing these two taxa into one group is based on molecular data sources, including both nuclear DNA, mitochondrial DNA, and RNA. A key thing to remember is that the cyclostomes diverged from the gnathostome line in the Early Cambrian period, leaving a lot of evolutionary time for hagfishes and lampreys to accumulate phenotypic changes that may mask their relatedness.

Figure 3.7—The black hagfish, Eptatretus deani.

Figure 3.8—An aquarium full of sea lampreys, Petromyzon marinus.
There are about 76 extant species of hagfish, which are also known as “slime eels.” Hagfishes, which are strictly marine, can produce several liters of slime from glands in their skin when attacked by a predator—this process only takes roughly 100 milliseconds. This mucus-like slime quickly adheres and clogs fish gills, making it an effective deterrent (Figures 3.9 and 3.10). They are also known for performing a unique knotting behavior when they encounter a particularly tough piece of carcass: They wrap themselves into a knot, then pass this knot down the body, creating some extra force to leverage the chunk of carcass off. This is helpful, given that they lack jaws. These knots are also used to escape, remove mucus, and occasionally pull live prey (worms and crustaceans) from burrows. Some hagfishes may also absorb nutrients from their surrounding environment through their skin.

Figure 3.9—A hagfish that has tied itself into a knot.

Figure 3.10—The seal shark Dalatias licha (a–c) and the wreckfish Polyprion americanus (d–f) attempt to prey on the hagfishes. First, the predators approach their potential prey. Predators bite or try to swallow the hagfishes, but hagfishes have already projected jets of slime (arrows) into the predators’ mouths. Choking, the predators release the hagfishes and gag in an attempt to remove slime from their mouths and gill chambers.
The lamprey, of which there are about 38 extant species, can be found in both fresh and salt water. Most lampreys spend the majority of their lives in the larval stage, called an ammocoetes larva. The larvae eventually metamorphose into a juvenile or adult, depending on the species. The ammocoete and adult lamprey are extraordinarily different from each other. Ammocoetes hatch in freshwater, live within the substrate at the bottom of the water body, and are filter feeders. Adult lampreys are not filter feeders. Some species, referred to as brook lamprey, do not actually eat as adults and stay in their original bodies of freshwater. They mature into a sexually mature adult from metamorphosis, spawn (reproduce), and die. The remaining species are categorized as parasitic lamprey. They metamorphose into sexually immature juveniles, start parasitizing other fishes, and then continue to grow and mature into sexually mature adults (Figure 3.11). While some species of parasitic juveniles stay in freshwater, other species will migrate to salt water. However, both will return back to freshwater to spawn and then die.

Figure 3.11—The life stages of a parasitic lamprey. Bottom left: filter-feeding ammocoetes larvae; top: parasitic juvenile; bottom right: parasitic adult body form.
Gnathostomata
All of the other vertebrates fall under the Gnathostomata, which translates to “jaw mouth” (Figure 3.6). Historically, the only vertebrates that were included in this group were vertebrates with jaws—bony and cartilaginous fishes, two groups of fossil fishes (placoderms and acanthodians), amphibians, reptiles (including birds), and mammals. The cyclostomes plus extinct jawless fishes were previously united under the group “Agnatha” (“without jaws”). However, in the last few decades, new technologies and techniques for studying fossils and evolutionary relationships have added two extinct jawless vertebrate groups (conodonts and ostracoderms) to our “jawed vertebrates.” These two groups, plus the placoderm fishes, are collectively referred to as stem gnathostomes, with the “stem” designation reflecting that they are some of the earliest members of the Gnathostomata. As we move through the gnathostomes, you will see that some taxa will have a dagger icon (†) in front of the name. This symbol is used in biology to indicate that that taxon is completely extinct.
Box 3.2—Understanding Geologic and Evolutionary Time
Think about the oldest person you know and how long ago they were born. It probably seems pretty far away. Now think about how long ago World War II feels (1939–1945). What about the Napoleonic Wars (1803–1815)? The American Revolution (1765–1783)? Egypt’s Golden Empire (1560–1080 BCE)? The events we talked about in Chapter 1 of this book? All of those probably feel incredibly long ago. For humans, a handful of decades, let alone centuries, seems like an incredibly long time ago.
When we’re talking about the evolutionary history of the vertebrates, the history of life, or even the full history of the Earth, the number of years ago shifts by several orders of magnitude: millions and billions of years ago. Given how humans think about time, this is really difficult for people to wrap their minds around. If you read that the Earth is approximately 4.5 billion years old, can you really fathom that amount of time? Or, what if we add a new fact: Life is 3.7 billion years old? We can add another fact that often messes with people’s minds: We are closer in time to Tyrannosaurus rex (extinct 65 million years ago) than T. rex was to Stegosaurus (extinct 145 million years ago).
One way that we deal with trying to reconcile geologic and evolutionary time in our heads is to talk about geologic periods and eras instead of how many millions of years ago. For example, “Jurassic” is a geologic period from 201 to 145 million years ago (abbreviated as Ma or MYA)—this is the period that Stegosaurus lived in. The Jurassic period, along with the Triassic and Cretaceous periods, make up the Mesozoic era. We can similarly divide up the time before and after the Mesozoic era into various periods and eras. Working with the names of periods and eras, and knowing the relationship of those periods and eras to one another, is a useful way of keeping the timeline straight. We call this system the geologic time scale (Figure 3.12). Look at Figure 3.12 and find the Mesozoic era. Which of the three periods in this era did T. rex live in? You will see some of these periods and the associated times they cover throughout this book.

Figure 3.12—The geological time scale for the Phanerozoic Eon. Oldest is at the bottom; youngest is at the top. Dates are based on the Geological Society of America’s geologic time scale, V. 6.0 (2022). Note that the Phanerozoic is only the most recent eon in geologic history.
This still doesn’t solve the problem of scale, though—how much a million and a billion are. For that, we usually turn to analogy. In this case, we are going to think about the face of a clock (Figure 3.13). Assume that the Earth’s origin (4.5 billion years ago, abbreviated as 4.5 Ga) is at 12:00. Life originates around 2:00. Eukaryotes (organisms with a nucleus and membrane-bound organelles in their cells) don’t show up until 6:30. Animals appear at around 10:00, mammals sometime after 11:00, and hominins (a group that includes humans) at 11:59:59—in the last second.

Figure 3.13—A projection of Earth’s 4.5-billion-year history onto a clock. Ma = millions of years ago, Ga = billions of years ago. Notice that the Phanerozoic Eon (which we saw in Figure 3.12) doesn’t begin until 541 Ma.
The gnathostomes have several features that unite them. These include
- The presence of a sclerotic ring—a bony element near the eye that forms due to ossification of the sclerotic capsule. You can see this clearly in the eye region of Dunkleosteus in Figure 3.18.
- Paired pectoral appendages that have supporting skeletal structures—your arms are a great example, but so are the pectoral fins in fishes.
- Dermal bone—a specific type of bone that forms via intramembranous ossification, a fancy way of saying bone that forms within a membrane instead of replacing a cartilage template.
- Dental tissues—those that make up true teeth, such as dentin and enamel.
- Perichondral ossification (or some sort of calcification) of the endoskeleton—unlike intramembranous ossification, this type of bone formation starts with cartilage acting as a template for the bone tissue to replace.
- Cellular bone—this is a particular type of bone that is formed by osteoblasts (bone-forming cells) within a bony matrix. Acellular bone (without osteoblasts) currently exists in some bony fishes, but your bones are decidedly cellular.
Note that this is only a partial list of gnathostome characteristics. There are other specific organs and skeletal structures as well, but these six will come into play shortly.
†Conodonts
The conodonts, which lived in the marine realm from the Cambrian through the Late Triassic, are a bit of a paleontological mystery. Very few whole-body fossils exist, but those rare body fossils reveal more chordate and vertebrate features: notochords, segmented trunk muscles, paired sensory organs, a caudal fin, and gill pouches (Figure 3.14). The vast majority of the fossil record of these extinct vertebrates is toothlike elements made of apatite, a mineral composed of phosphate and calcium, which are no larger than 1 mm long (Figure 3.15). Using apatite as a skeletal component is another vertebrate feature, and the array of tissues in these tooth elements is very similar to dental tissues in other gnathostomes (see gnathostome characteristic 4; Figure 3.16). However, it is not clear whether these are truly homologous with other gnathostome tooth tissues, and thus their relationship to other vertebrates is cloudy. For now, conodonts are considered to be stem gnathostomes.

Figure 3.14—A reconstruction of a conodont.

Figure 3.15—Tooth elements from the Ordovician conodont Iowagnathus grandis.

Figure 3.16—Tooth elements from the conodont Hindeodus parvus arranged in the order that they occur in the mouth. Elements at the top of the diagram occur anteriorly, while those at the bottom are posterior to those at the top of the diagram. Tooth element M is the most lateral tooth element, while tooth element S0 is the most central.
†The “Ostracoderms”
The “ostracoderms” are a variable group of jawless fishes that lived from the Ordovician through the Devonian (Figure 3.17). We’ve put their name in quotes because they represent several evolutionary lines, as opposed to one particular taxonomic group. All the “ostracoderms” have some sort of bony armor, but its shape and size vary across the groups. For example, there are two groups, the Cephalaspidomorphi (“head shield form”) and Pteraspidomorphi (“wing shield form”) that have extensive head shields made out of bone. The Thelodonti and Anaspida are two other groups that have small scales instead of large bony shields. This group of largely jawless fishes seems to be an odd thing to include in the Gnathostomata, a taxon named for having jaws. However, when one considers characteristics beyond jaws, the inclusion makes more sense. All ostracoderms have some sort of mineralized, dermal bone skeleton. Some ostracoderms have all six of the gnathostome characteristics highlighted above, plus a few more, indicating that these particular gnathostome features appeared prior to jaws! For example, two groups of cephalaspids, the Galeaspida and Osteostraci, are the closest to the jawed gnathostomes. The galeaspids have a neurocranium (braincase) composed of mineralized cartilage, while the osteostracans have one composed of perichondral bone (gnathostome characteristics 5). The osteostracans also have paired pectoral fins and cellular bone (gnathostome characteristics 2 and 6), making them the closest jawless fish group to the jawed vertebrates (Figure 3.6).

Figure 3.17—Fossil jawless and jawed fish. (A) Pteraspis, a pteraspidomorphan. Two Cephalaspidomorphans: (B) Hemicyclaspis (Osteostraci) and (C) Pterygolepis (Anaspida). A, B, and C are all ostracoderms (jawless, armored fish). (D) Climatius is a fossil acanthodian with a jaw.
†The “Placoderms”
The “placoderms,” which lived from the early Silurian to the late Devonian, are another group of fishes with extensive head armor and varying morphologies. The most well-known placoderm group (at least to those who frequent natural history museums or are fans of the movie Ponyo) is the arthrodires, specifically the very large Dunkleosteus (Figures 3.18 and 3.19). Most arthrodires were active predators with sharp dermal plates on the jaws. However, most orders of placoderms were benthic (bottom-dwelling) forms such as Bothriolepis (Figure 3.20). They differ from the ostracoderms due to several characteristics that they share with other gnathostomes:
- Paired pelvic appendages that have supporting skeletal structures—your legs are a great example, as are the pelvic fins of many fishes.
- Five or fewer branchial arches—in fishes, these are the elements that support the gills. You will see in later chapters where these gill arches have gone in terrestrial vertebrates.
- Jaws that operate dorsoventrally—this is different from the mouths of some conodonts and the cyclostomes, which move laterally. As you will read in Chapter 8, there are two main elements, the palatoquadrate (upper jaw) and Meckelian element (lower jaw). These elements will be greatly modified as we move through evolutionary time, including one of those elements being incorporated into the mammalian ear.
- Gnathostome jaws also bear dermal denticle plates, which act as teeth. The question of what defines something as a true tooth is currently being debated by scientists, as much of the helpful soft tissue and developmental data are not available in the fossil record; thus we cannot say whether placoderm teeth are truly homologous with other gnathostome teeth.

Figure 3.18—Dunkleosteus terrelli, an arthrodire placoderm from the Devonian of Cleveland, Ohio. This is a cast of one of the most complete fossils we have for this fish.

Figure 3.19—Various size estimates for Dunkleosteus terrelli, with a human shown for scale. Because we have only found the head and part of the shoulder girdle, we have to hypothesize the size and shape of the body.

Figure 3.20—A reconstruction of the Devonian antiarch placoderm Bothriolepis canadensis.
We’ve placed the name “placoderms” in quotes because it’s not clear whether the placoderms should be put together into one group or if they represent several evolutionary lineages. Historically, these fishes were grouped together because they are where we first see jaws in the fossil record. We also know that all placoderms share particular features of the neurocranium that other gnathostomes do not and that they may share a unique version of dentine not seen in other gnathostomes. However, recent studies have also shown that variations in other neurocranium features, the articulation of the hyoid arch with the neurocranium, and inner ear features support multiple evolutionary lineages. There is also a group of placoderms that are called “maxillate,” as they have a premaxilla, maxilla, and dentary instead of just a palatoquadrate and Meckelian element. More simply put: We have not figured out what to do with the placoderms other than agreeing they are stem gnathostomes.
Eugnathostomata
The remainder of our gnathostomes fall under the Eugnathostomata (“true gnathostomes”), reflecting that these organisms all have the features we’ve already discussed as gnathostome characteristics, plus true teeth and several cranial characteristics related to the skull and eye. While teeth will be discussed more in Chapter 8 and we mentioned some of the issues defining teeth above, we will point out that among our extant eugnathostomes, true teeth are in part defined by developing within a dental lamina—an epidermal soft tissue that does not fossilize readily—in a particular pattern.
The eugnathostomes include the Chondrichthyes (cartilaginous fishes), Osteichthyes (bony fishes, which technically include amphibians, reptiles, birds, and mammals), and an enigmatic group of extinct fishes called acanthodians (see Figure 3.6 if you need a reminder of what the vertebrate phylogenetic tree looks like).
Chondrichthyes
The Chondrichthyes (“cartilage fish”) have been around for over 400 million years, meaning that they have been here longer than trees, insects, and the rings of Saturn. The chondrichthyans are divided into two groups: the Elasmobranchii (“plate gills”), which includes sharks, skates, and rays; and the Holocephali (“complete heads”), which includes the chimeras, rat fishes, and ghost sharks (which are not sharks; Figure 3.21). Across their evolutionary history, the Chondrichthyes have varied considerably in morphology and lifestyle, including everything from the six-inch extant dwarf lantern shark Etmopterus perryi to the extinct “buzzsaw shark” Helicoprion (which is actually a holocephalan that was potentially 20 ft. long) to the massive extinct Otodus megalodon that was at least 18 meters long (Figures 3.21 and 3.22). The biggest extant fish, the whale shark, can be up to 40 feet long and feeds on krill and other small organisms (Figure 3.21).

Figure 3.21—Various extant chondrichthyans. Top: Rhincodon typus, the whale shark. Middle: (left) Rhinoptera bonasus, the cownose ray; (right) Etmopterus perryi, the dwarf lantern shark. Bottom: (left) Hydrolagus colliei, the spotted ratfish; (right) Galeocerdo cuvier, the tiger shark.

Figure 3.22—Top: (left) a tooth from Otodus megalodon; (right) a tooth whorl from Permian-aged Helicoprion. Bottom: a reconstruction of Helicoprion.
As the name implies, the chondrichthyans have endoskeletons made of cartilage, not bone. This specific cartilage is unique to the chondrichthyans in that it is tessellated prismatic calcified cartilage (Figure 3.23). Calcified refers to the addition of calcium phosphate hydroxyapatite to the cartilage. Tesserae are small blocks of calcified cartilage that overlie an unmineralized cartilage core (thus we say the cartilage is “tessellated”), whereas prismatic refers to the pattern of calcification that allows the cartilage to refract light when sliced thinly and examined under a microscope. While this unique cartilage is more durable than the cartilage that is, for example, in your nose, it still is not as durable as bone when it comes to surviving the fossilization process. Thus, the vast majority of the evolutionary history of chondrichthyans is a story told largely in teeth and denticles (scales), which are composed of more durable enameloid and dentine, as well as vertebrate and fin spines, which are more heavily mineralized than other cartilaginous elements. This means that the earliest chondrichthyan fossils may be isolated denticles from 450 million years ago. The earliest chondrichthyan teeth are about 420 million years old, and the oldest body fossil is 394 million years old.

Figure 3.23—General organization of the primary mineralized skeletal tissue in sharks and rays, tessellated cartilage. (a) Cleared and stained butterfly ray skeleton (Gymnura sp.). Specimen is young and not yet fully mineralized; blue color shows the cartilage of the skeleton, which will form a mineralized layer later in life. (b, c) Structure of tessellated cartilage of sharks and rays, comprising mineralized tiles (tesserae) covering the skeletal cartilage. coll = collagen; P = perichondrium; T = tesserae; UC = unmineralized cartilage.
†The Acanthodians
The acanthodians (“spiny” or “prickly”), which lived from the Silurian through the Permian, have often been referred to as the “spiny sharks” due to their characteristic ventral fin spines (Figure 3.17). While most recent studies indicate that they represent multiple evolutionary lineages, studies do not agree on where they should be placed with regard to their relationships with the other eugnathostomes. They seem to share some features specific to the chondrichthyans, such as a largely cartilaginous skeleton, tesserae, and a few braincase features. However, other features are more typical of the osteichthyans, such as the bony bases of their fins, their scale structure, and having enamel (which has an epidermal origin) instead of enameloid (which has a dermal origin). As we saw with the chondrichthyans, a cartilaginous skeleton is not conducive to fossil preservation; as such, the fossil record of this group is sparse, which makes it harder to resolve their relationship with other eugnathostomes. When all these characteristics are taken together and subjected to phylogenetic analyses, the consensus seems to be that the acanthodians are stem chondrichthyans, even though how those acanthodians are related to each other is still unclear.

Figure 3.24—A Devonian-aged acanthodian, Diplacanthus striatus.

Figure 3.25—Reconstructions of various acanthodians clockwise from top center: Acanthodes bronni, Climatius reticulatus, Ischnacanthus gracilis, Parexus recurves, Gyracanthus formosus, Cheiracanthus murchisoni, and (center) Diplacanthus crassissimus.
Osteichthyes
The Osteichthyes (“bony fish”) have been around for almost as long as the chondrichthyans, first appearing in the fossil record in the Silurian period (roughly 425 million years ago). As the name implies, the osteichthyan skeleton is composed mostly of bone, and many of the features that characterize the osteichthyans are indeed bony. For example, the braincase and jaws are surrounded by a particular set of dermal bones. Likewise, the palate and a large component of the pectoral bones are also composed of dermal bones. Fin rays, called lepidotrichia, are composed of bone too. There is a long list of other characteristics involving the braincase, other skeletal elements, and nerves that we will not go into here. The take-home message is that there are many characteristics that define osteichthyans, and most of them revolve around the skeleton.
The osteichthyans include two groups (Figure 3.6). The first, the Actinopterygii (“ray fins,” named for their fin structure, as the fin is mostly composed of the long-fin rays called lepidotrichia), are what you probably would think of if we asked you to think of a fish (Figures 3.26 and 3.27). These include over 50% of the extant vertebrates. They include minnows, goldfish, gar, paddlefish, eels, flounders, and seahorses. Extant actinopterygians range in size from the 8-mm-long carp-like fish Paedocrypris to the giant oarfish Regalecus glesne at up to 36 feet long.

Figure 3.26—The morphological diversity of extant actinopterygians.

Figure 3.27—The pectoral fin of the actinopterygian Epinephelus merra, the honeycomb grouper.
The actinopterygians are typically divided into three groups based on both genetic and morphological data. The first is the Cladista, composed of the bichirs and reedfish (Figure 3.28). The extant members of this freshwater group live in Africa. The second group is the Acipenseriformes (“sturgeon form”), which includes sturgeons and paddlefish (bottom left of Figures 3.26 and 3.29, respectively). All other actinopterygians fall under the Neopterygii (“new fin”). Unlike the other groups of actinopterygians, the neopterygians have a caudal fin that has generally symmetrical lobes as well as forty other common features that are beyond our introduction here. The neopterygians are further divided into the Holostei (“whole bone,” gars and bowfin; Figures 3.26 [bottom right] and 3.30, respectively) and the Teleostei (everything else, Figure 3.26 except bottom row). The holosteians, while looking very different from one another, are united by having two vertebral centra fused to the occipital condyle of the skull and 15 other features. The identity of those 15 features is not important for our purposes, but it demonstrates that these fishes have more in common than external appearances imply.

Figure 3.28—Polypterus senegalus, the Senegal bichir, is a member of Cladista.

Figure 3.29—Polydon spatula, the American Paddlefish, is a member of the Acipenseriformes. Another acipenseriform, a sturgeon, can be seen in the bottom left corner of Figure 3.26.

Figure 3.30—Amia calva, the bowfin, is a member of the Holostei. Another holosteian, a gar, can be seen in the bottom right corner of Figure 3.26.
The Teleostei (“complete bone”) encompass the majority of the extant actinopterygian species, approximately 35,000, and are united as a group by an assortment of skeletal and soft-tissue features. A number of innovations have led to their massive evolutionary success. They are the only group of fishes that has duplicated their entire genome, which, if you recall from the section on Hox genes, can release constraints and allow for evolutionary experimentation. In particular, the evolution of oral jaw protrusion (Figure 3.31) and pharyngeal jaws (an independent set of jaws derived from the gill arches; Figure 3.32) opened up new feeding niches that were previously unavailable. Changes to the fin shapes and locations along the body further allowed teleosts to exploit a variety of new habitats.

Figure 3.31—A largemouth bass, Micropterus salmoides, showing jaw protrusion as the fisher opens the bass’s mouth.

Figure 3.32—Schematic drawing (lateral view) of a generalized cichlid (teleost) cranial skeleton, showing the relative location of the oral jaws (purple) and the pharyngeal jaws of the most posterior arch (blue).
The second osteichthyan group is the Sarcopterygii (“flesh fins”). Unlike the actinopterygians, only a small portion of their pectoral and pelvic fins are composed of lepidotrichia. The base of the sarcopterygian pelvic and pectoral fins are composed of only one bone (the metapterygium), whereas in actinopterygians there are four (collectively called basals). The sarcopterygian fins are also fleshy with muscle and connective tissue, unlike the actinopterygians. Together, these fin features give the sarcopterygians their common name of “lobe-finned fishes.”
The living sarcopterygians include three groups (Figure 3.34). The first is the Actinistia, which comprises the coelacanths (Figures 3.33 and 3.35). The extant coelacanths were only brought to light in 1938 by Marjorie Courtenay-Latimer, a South African museum naturalist who received a call from a fisherman who brought in an unusual fish he thought she would be interested in. This was particularly noteworthy because coelacanths had been known from the fossil record for much longer and were thought to have been extinct for 80 million years. The genus of extant coelacanths is named Latimeria in her honor. The two extant species, which can be up to 6.5 feet long, live in the deep sea of the West Indian Ocean and Indonesia and can live for almost a century. However, the actinistians were far more species-rich in the Paleozoic era and showed far more morphological diversity than we’ve seen since then. The extant coelacanths superficially resemble some of the extinct forms but have their own particular bone pattern in their pelvic and pectoral fins.

Figure 3.33—Top: A sarcopterygian fish, an extant coelacanth. Note the fleshy lobed pectoral fins (red arrow), and compare that to the actinopterygian ray-fin in Figure 3.27. Bottom left: The skeletal elements of an actinopterygian pectoral fin (from the bowfin Amia). Bottom right: The skeletal elements of a sarcopterygian pectoral fin.

Figure 3.34—From top to bottom and left to right, examples of sarcopterygians: †Guiyu oneiros, Latimeria chalumnae, Neoceratodus forsteri, and †Panderichthys rhombolepis.

Figure 3.35—Marjorie Courtenay-Latimer and coelacanth after its discovery.
The second group is the Dipnoi (“two lungs”), or lungfishes; they are named for their paired lungs, which are modified swim bladders that can indeed absorb oxygen (Figure 3.36). They also share a unique arrangement of tooth plates, which are only located on the palate, and firmly joined halves of the mandible. Like the coelacanths, lungfishes were far more diverse in the past than they are today. Only three genera are still extant—one each in Africa, South America, and Australia—and it’s important to remember that they have changed quite a bit since their lineage split away from the other sarcopterygians.

Figure 3.36—A marbled lungfish, Protopterus aethiopicus, which lives in Africa.
The final group of sarcopterygians is the Tetrapodomorpha. The Tetrapodomorpha includes a line of fishes that existed from the Devonian through the Early Permian but also includes the evolutionary lineage of the Tetrapoda, the terrestrial vertebrates (amphibians, reptiles, birds, and mammals). We will talk about the fishes here and then treat the Tetrapoda separately in the next section. The “fish contingent” of the Tetrapodomorpha contains four evolutionary lineages that cover the transition from fully aquatic fishes to the “fishapod” transitionary forms such as Eustenopteron and Tiktaalik (Figures 3.37 and 3.38). You will learn about these fishes in greater detail in subsequent chapters, as they’ve played an important role in understanding one of the biggest evolutionary shifts in vertebrate history: from aquatic to fully terrestrial environments. For now, we know that the tetrapodomorphs, including the fully terrestrial forms like us, are united as a group because of the presence of internal nostrils called choanae. However, it seems that some lungfishes have choanae as well, supporting the idea that lungfishes are more closely related to tetrapodomorphs than the coelacanths but with the problem of not having a uniting feature for all the tetrapodomorphs.

Figure 3.37—A cast of the “fishapod” Tiktaalik roseae at the Field Museum of Natural History.

Figure 3.38—A reconstruction of Tiktaalik roseae.
3.3 Tetrapoda
The rest of the vertebrates fall under the Tetrapoda. This includes amphibians, reptiles, birds, and mammals (Figure 3.39). Tetrapoda translates to “four feet,” pointing to their defining characteristic: The front and hind limbs have digits (i.e., fingers and toes). The tetrapodomorph “fishapods” don’t have digits on the end of their fins. There are, of course, exceptions among our living tetrapods, including the limbless snakes and marine mammals such as dolphins and whales. But as we’ve said before, we have to remember that these extant tetrapods have had a lot of time for evolutionary changes. Other defining characteristics include specialized articulations between vertebrae to support the backbone against gravity and modifications to the ulna (a bone in the forearm) to support muscle attachment.
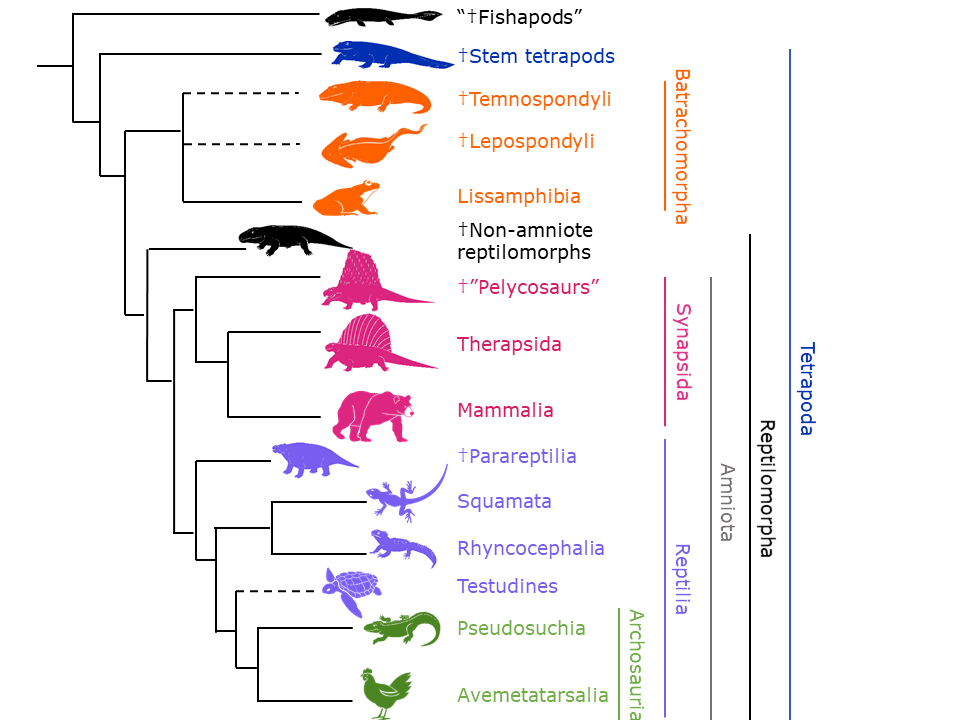
Figure 3.39—A phylogenetic tree of the Tetrapodomorpha. Dotted lines indicate that the placement of the branch is uncertain. The dagger (†) indicates that all members of that taxonomic group are extinct.
The earliest tetrapods (stem tetrapods), such as Ichthyostega, made their appearance in the late Devonian period and looked superficially very similar to the “fishapods” like Tiktaalik but with feet (which are really fins with digits) instead of fins without digits. They retained several fish characteristics, such as their gills and lateral line canals (a sensory system that only works in water; see Chapter 20). As time progressed, the stem tetrapods lost more and more of their fishy features as adaptations allowed them to become more terrestrial.

Figure 3.40—A simplified phylogenetic tree showing the fish-tetrapod transition, illustrating some of the major anatomical changes.
Batrachomorpha
The Batrachomorpha (“frog forms”) encompasses two extinct groups of tetrapods, called the temnospondyls and lepospondyls, and the group that includes the extant amphibians and their extinct relatives, the Lissamphibia. All groups have four or fewer digits on their hands. The †Temnospondyli (“cut vertebra”) have been found in fossiliferous deposits from the Mississippian period through the Early Cretaceous around the world (Figures 3.41 and 3.42). They were quite successful and diverse in size and shape, and they comprised over 160 genera across both land and water. Most temnospondyls were carnivores. The †Lepospondyli (“husk vertebra”) lived from the Mississippian to the late Permian (Figure 3.43). These were not quite as diverse as the temnospondyls, tended to be relatively small and elongated, and included some burrowers with reduced limbs. It is not clear whether the Lissamphibia lineage came from one of the temnospondyl evolutionary lines, if it came from one of the lepospondyl lines, or if the individual lissamphibian lineages arose from multiple temnospondyl and lepospondyl lines. There are some questions about the timing of lissamphibian fossils versus likely temnospondyl ancestors, as the earliest lissamphibian fossils are also from the Mississippian period.

Figure 3.41—A variety of early temnospondyl skulls from the clade Edopoidea, showing some of the diversity in size and shape within the †Temnospondyli.

Figure 3.42—The temnospondyl Eryops. The top image is the full skeleton, and the bottom image is a close-up of the snout and teeth.

Figure 3.43—A skeleton cast and reconstruction of the lepospondyl Diplocaulus.

Figure 3.44—A reconstruction of the lepospondyl Brachydectes newberryi.
The Lissamphibia include the extant amphibians and their extinct relatives. The extant Lissamphibia, representing approximately 20% of extant tetrapods, is composed of three diverse groups: the Caudata (salamanders), Gymnophiona (legless amphibians also known as caecilians), and Urodela (frogs), and these extant groups all originate in the Jurassic period (Figure 3.45). Both molecular and morphological data support the idea that the urodeles and caudates are more closely related to each other than to the gymnophionans. Even though extant lissamphibians have very different morphologies, they are united by the shape of their teeth (two-cusped and with a base composed of dentine between the jaw bone and tooth crown), the presence of a particular eye muscle called the levator bulbi that serves to elevate the eye, having particular skin glands (see Chapter 6), and many skeletal features. As the name “amphibian” indicates, these animals are tied to both water and land (“amphi” = both).

Figure 3.45—Extant lissamphibians. Top left: Australian green tree frog Litoria caerulea. Bottom left: Mexican burrowing caecilian Dermophis mexicanus. Right: Eastern newt Notophthalmus viridescens in the red eft phase.
Reptilomorpha
The Reptilomorpha (“reptile shape”) includes the remaining vertebrates, the amniotes, as well as a few reptile-like tetrapods that aren’t amniotes. We will treat the amniotes in their own section. The nonamniote reptilomorphs (Figure 3.46), which lived from the Mississippian through Permian periods, share a handful of features such as narrow premaxillae (part of the upper jaw) and a particular pattern of joint numbers as you move across the toes (we are 2-3-3-3-3, but the nonamniote reptilomorphs are 2-3-4-5-4). As one might expect, many of the nonamniote reptilomorphs look like a cross between an amphibian and a reptile and include aquatic, semiaquatic, and terrestrial forms.

Figure 3.46—Reconstructions of nonamniote reptilomorphs. Top: Seymouria baylorensis. Bottom: Archeria crassidiscus.
Amniota
The Amniota are named after their most characteristic shared feature: the amniotic egg. This special egg has four extraembryonic layers (“outside of the embryo”), the amnion, chorion, yolk sac, and allantois. You will learn a great deal more about this egg and why it helped the amniotes conquer terrestrial environments in Chapter 17. The amniotes are divided into two groups, the Synapsida and the Reptilia (sometimes referred to as the Sauropsida). These two groups diverged from each other during the Carboniferous period. Historically, the amniotes were separated into various groups based on the location of various openings called temporal fenestrae (“windows”) in the skull that are used for the attachment of jaw-closing muscles: Synapsida possessed a single temporal fenestra on each side of the skull (the lateral temporal fenestra), Anapsida possessed none, and Diapsida possessed two (upper and lower temporal fenestra). However, the use of molecular data and data from other skeletal features has reminded us that organisms change over evolutionary time, and many amniotes secondarily lost or regained various fenestra. While the Synapsida and Diapsida remain valid groupings, the anapsids have been distributed across various other groups.
Synapsida
The Synapsida include a large number of extinct reptiles, including the sail-backed Dimetrodon (which is not a dinosaur!) and several other “mammal-like reptiles,” as well as the Mammalia. These groups are generally united by possessing a single temporal fenestra on each side of the skull, although there are several other skull features as well. The Synapsida arose about 318 million years ago, during the Pennsylvanian period. The synapsids can be split into two groups: the Therapsida, which gave rise to the mammals, and a group of basal synapsids that are generally called the “†pelycosaurs” but are not a particular evolutionary grouping. The pelycosaurs (Figure 3.48) were the main terrestrial vertebrates from the Pennsylvanian through the Early Permian period and were extinct before the end of the Triassic period. The pelycosaurs were more than just Dimetrodon—they included herbivores, carnivores, and piscivores (fish eaters), and many did not have sails. Their body plans were fairly similar to some of the nonamniote tetrapods, with sprawling limbs, long tails, and a homodont dentition (all teeth were roughly the same shape).

Figure 3.47—The bones in the skull and jaws of the synapsid Dimetrodon, shown in lateral view. You will learn a lot more about these bones in Chapter 8.

Figure 3.48—Various pelycosaurs. Top left: Spenacodon ferox. Top right: various Dimetrodon species. Bottom row: reconstruction and skeleton of Edaphosaurus pogonias.
The therapsids (Figure 3.49) were the dominant terrestrial vertebrates from the Middle Permian until the mass extinction event at the end of the Permian period. There are approximately six different groups of therapsids, and they span a wide range of morphologies and sizes. Unlike the pelycosaurs, most therapsids were herbivores, although there were several carnivorous species, such as the gorgonopsians, which had very large canines and could open their mouths more than 90 degrees (Figure 3.50). The therapsids also show evidence of more active lifestyles, indicating a potential shift toward endothermy (body temperature is internally generated and regulated). Cynodontia is the particular group of therapsids that led to the mammals (and technically includes the Mammalia). Cynodonts first appeared in the fossil record toward the end of the Permian period and persisted until the Triassic period. We see a few mammalian features in the cynodonts, such as particular muscular markers on the skull and a heterodont (Greek for “different teeth”) dentition. However, it’s worth noting that various groups of therapsids that are not cynodonts independently evolved characteristics that we consider “mammal-like” as well, including a complete secondary palate, precise occlusion of teeth in the upper and lower jaws, and particular bone patterns in the hands and feet.

Figure 3.49—Reconstructions of five therapsids that illustrate the diversity of body forms. Clockwise from top left: Inostrancevia, Alopecognathus, Ostehria, Moschops, and Castorocoda.

Figure 3.50—Skull of the gorgonopsid Gorgonops.

Figure 3.51—The cynodont Procynosuchus. Note that the limbs are under the body instead of a more reptilian sprawling posture.
The Mammalia first appeared in the fossil record during the Late Triassic period. These tended to be small, shrew-sized insectivores at first but started diversifying in the Late Jurassic period. Mammals, including you, are united as a group by the presence of milk-producing glands called mammary glands and hair. Unfortunately, these are difficult to preserve in the fossil record, so we often look for shared skeletal features as well. Specifically, these are the presence of three bones (incus, malleus, and stapes) in the middle ear, a lower jaw composed of only one bone (dentary), and the formation of the jaw joint between the dentary and a bone in the skull called the squamosal (see Figure 3.47). We will follow these skeletal transitions in more detail in several other chapters.
Extant mammals have diversified into over 6,600 species, from the bumblebee bat (1 inch long) to the largest animal on Earth, the blue whale (110 ft. long). All extant mammals are endothermic, meaning they produce their own body heat, and they are found in just about every available habitat. We divide extant mammals into three groups: the Monotremata (egg-laying mammals), Metatheria (marsupials), and eutherians (all other mammals, including you); the latter two are united together as the Theria. Each group is generally defined by a particular reproductive strategy, which you learn more about in Chapter 17.
Extant monotremes (Figure 3.52), the egg-laying mammals, are currently restricted to Australia, Tasmania, and New Guinea. They include only the platypus (Ornithorhynchus anatinus) and echidnas (four species); however, they were more diverse during the Late Cretaceous and included the largest mammals at that time (weighing up to 20 kg). One fossil has been found in Patagonia, indicating that they may have lived in Antarctica as well (there was no other way for them to get to South America, as these continents were united as the supercontinent Gondwana at that time). The Monotremata diverged from the lineage leading to the Theria (Metatheria + Eutheria) sometime between the Triassic and Early Jurassic periods, but studies using various molecular techniques do not agree on the specific timing. The earliest monotreme fossils are from the Early Cretaceous.

Figure 3.52—Extant monotremes. Clockwise from top left: platypus, short-beaked echidna, eastern long-beaked echidna, western long-beaked echidna.
The extant 399 species of metatherians are represented by the marsupials (Figure 3.53), most of which are restricted to Australia, the islands near Australia (collectively called Wallacea after Alfred Russel Wallace), and South America. These include kangaroos, koalas, opossums, bandicoots, quokkas, and Tasmanian devils. North America only boasts one extant species of marsupial, the Virginia opossum Didelphis virginiana, a recent immigrant that only arrived 3 million years ago when the isthmus of Panama formed. The restriction of marsupials to the Southern Hemisphere reflects the geological history of the Gondwana and its isolation from Laurasia (another supercontinent, composed of North America and Eurasia). During this period of isolation, marsupials formed communities as diverse as we see in today’s eutherian-dominated mammalian communities. These included large predators, insectivores, browsers, and more.

Figure 3.53—Extant metatherians. Clockwise from left: kangaroo, Virginia opossum, long-nosed bandicoot, monito del monte, Tasmanian devil.
The eutherians (often called “placental mammals,” which misleadingly suggests that the marsupials don’t have a placenta) diverged from the marsupials in the middle of the Jurassic period. The extant eutherians include most of the living mammals, roughly 6,200 species, including us (Table 3.1; Figure 3.54). The earliest fossils come from Mongolia and Montana and are about 105–110 million years old. They tended to be rather small, but once the nonavian dinosaurs went extinct at the end of the Cretaceous period, most of the extant orders had made an appearance within 15 million years. These were by no means the only orders present at that time; another 16 or so orders also have come and gone. Most of our extant orders of mammals originated in the Northern Hemisphere, with the exception of the Xenarthra (anteaters and their kin in South America).
Table 3.1—A list of the various extant eutherian orders, plus an estimate of the number of extant species
Data from the American Society of Mammalogists Mammal Diversity Database
|
Order name |
# of extant species |
What is in that order |
|
Afrosoricida |
55 |
Golden moles, otter shrews, tenrecs |
|
Artiodactyla |
360 |
Even-toed hoofed mammals: deer, bovines, antelope, dolphins, whales, hippos |
|
Carnivora |
310 |
Cats, canines, raccoons, otters, weasels, hyenas, bears, pinnipeds, mongooses |
|
Chiroptera |
1,466 |
Bats, flying foxes |
|
Cingulata |
22 |
Armadillos |
|
Dermoptera |
2 |
Colugos |
|
Eulipophyla |
584 |
Hedgehogs, shrews, moles |
|
Hyracoidea |
6 |
Hyraxes |
|
Lagomorpha |
111 |
Rabbits, hares, pikas |
|
Macroscelidea |
20 |
Elephant shrews |
|
Perissodactyla |
18 |
Horses, zebras, rhinos, tapirs |
|
Pilosa |
17 |
Anteaters, sloths |
|
Pholidota |
8 |
Pangolins |
|
Primates |
517 |
Apes, monkeys, lemurs, humans |
|
Proboscidea |
3 |
Elephants |
|
Rodentia |
2,680 |
Mice, rats, squirrels, beavers, capybara, chinchillas, hamsters |
|
Scandentia |
23 |
Treeshrews |
|
Sirenia |
4 |
Manatees, dugongs |
|
Tubulindentata |
1 |
Aardvark |

Figure 3.54—A variety of extant eutherian mammals. Left column, from top to bottom: common vampire bat Desmodus rotundus, eastern gray squirrel Sciurus carolinensis, plains zebra Equus quagga, aardvark Orycteropus afer, humpback whale Megaptera novaeangliae, and black and rufous elephant shrew Rhynchocyon petersi. Middle column: humans Homo sapiens, ground pangolin Manis temminckii, Sunda flying lemur Galeopterus variegatus, West Indian manatee Trichechus manatus, European hedgehog Erinaceus europaeus, nine-banded armadillo Dasypus novemcinctus. Right column: southern elephant seal Mirounga leonina, Asian elephant Elephas maximus, reindeer Rangifer tarandus, giant anteater Myrmecophaga, giant panda Ailuropoda melanoleuca, and American pika Ochotona princeps.
Reptilia
The Reptilia (also known as the Sauropsida) contain what we typically think of as reptiles (snakes, lizards, crocodylians), as well as dinosaurs, pteranodons, marine reptiles like ichthyosaurs, and birds. Despite diverging from the synapsids approximately 315–330 million years ago, the Reptilia did not become the dominant terrestrial fauna until the Mesozoic era. The Reptilia are often split into two main groups, which do not include a small number of stem Reptilia: the †Parareptilia, which were the dominant group of reptiles in the Permian and the Early Triassic periods, and the Eureptiles, which contain our extant reptiles and were the dominant reptiles of the Mesozoic. Both of these main groups are considered part of the Diapsida. The parareptiles included a wide variety of reptiles that occupied several ecological niches across the globe, including small carnivores, aquatic predators, and large herbivores (Figure 3.55).

Figure 3.55—Parareptilia. Top: Bradysaurus baini. Bottom left: Mesosaurus. Bottom right: Sclerosaurus armatus.
In addition to some stem eureptiles, the Eureptilia include all the extant reptiles (including birds) and several notable extinct reptiles (nonavian dinosaurs, pteranodons, and several marine reptile groups). Many of the familiar extant reptiles fall into the Lepidosauria, which has over 11,000 extant species; these are divided into the Squamata (snakes, lizards, and a different group of legless reptiles called amphisbaenians) and Rhynchocephalia (one extant species, the tuatara, which lives in New Zealand). These two groups likely diverged sometime in the Early Triassic. The lepidosaurs share particular lower limb structures, including a unique structure of the knee joint, the ability to self-amputate their tails (caudal autonomy) as a self-defense mechanism, and some soft-tissue features that you will learn about in the chapters on urogenital structures (Chapters 16 and 17).
Despite only having one living species, the Rhynchocephalia (“beak heads”) were globally abundant during the Late Triassic and Jurassic periods (Figure 3.56). Most of these were small, terrestrial, and lizard-like, as is the extant tuatara Sphenodon. However, there were also aquatic versions, called pleurosaurs, that were elongated and had reduced limbs. At their most abundant, the Rhynchocephalia included herbivores, omnivores, carnivores, and insectivores.

Figure 3.56—Rhynchocephalia. Top: Pleurosaurus. Bottom: Henry, the world’s oldest Sphenodon.
Even though the extant Squamata (snakes, lizards, and their relatives; Figure 3.57) are largely restricted to terrestrial habitats with a few notable exceptions, their evolutionary history includes a global distribution (including the Arctic and Antarctic) and almost every terrestrial and aquatic environment. The finer details of the evolutionary tree and taxonomy of squamates are highly contested, as molecular, morphological, and developmental data are at odds with each other. However, the taxonomic groups generally include the Serpentes (snakes), Iguania (iguanas, Anolis lizards, chameleons, agamas, and more), Anguimorpha (varanids like monitor lizards and Komodo dragons, Gila monsters, glass lizards, and a few legless lizards), Scincoidea (heavily scaled lizards including the skinks), Lacertoidea (spectacled lizards, legless amphisbaenids, and some other lizards), and Gekkota (geckos). These are generally united by a pleurodont dentition (teeth are on the lingual, or tongue side, of the jaw), how kinetic (movable) their skulls are, and their foot structure. The extinct mosasaurs (Figure 3.58), large Mesozoic marine reptiles such as the Mosasaurus seen in the movie Jurassic World, are technically squamates, as they belong to the Anguimorpha.

Figure 3.57—A sampling of extant squamates. Top row, from left to right: Mexican blind lizard Anelytropsis papillosus, common flat-tail gecko Uroplatus fimbriatus, common chameleon Chamaeleo chamaeleon. Middle row: Sand lizard Lacerta agilis, rock monitor Varanus albigularis, grass snake Natrix natrix. Bottom row: Proctoporus chasqui (which doesn’t have a common name), red worm lizard Amphisbaena alba, common blue-tongued skink Tiliqua scincoides.

Figure 3.58—The skeleton and a reconstruction of the mosasaur Halisaurus.
We now leave the lepidosaurs to learn about a few more eureptile groups. The turtles and tortoises (Figure 3.59), or Testudines, are somewhat of an enigma in that their anatomy is so specialized that it makes understanding their evolutionary history difficult. Their most prominent feature is their shell, which is composed of a dorsal carapace and ventral plastron. Contrary to what cartoons may have led you to believe, the carapace and plastron are part of the skeleton, and turtles cannot exit their shells. The ribs and vertebrae are fused with several other bones (about 50 individual bones in total), and the pelvis and shoulders are highly modified to work with the shell. Extant turtles have no temporal fenestrae, nor do they have teeth. In other words, all the skeletal features we would normally use to figure out the relationship between turtles and other reptiles have been highly modified. As such, turtles have moved around the evolutionary tree of amniotes since people started to study them. The fossil record is not terribly helpful either; all of a sudden, there are recognizable turtles and no obvious transitional turtles. Molecular studies, paired with developmental data and more fossil work, have all helped us understand that turtles are indeed diapsids and have secondarily lost their temporal fenestrae. However, we’re not sure where in the Diapsida they fit, though the current accepted placement is as sister to the Archosauria (crocodylians and birds).

Figure 3.59—Extant Testudines. Clockwise from upper left: Red-bellied short-necked turtle (Emydura subglobosa), Indian flapshell turtle (Lissemys punctata), Hawksbill sea turtle (Eretmochelys imbricata), and Galápagos tortoise (Chelonoidis nigra). Note the diversity of body forms.

Figure 3.60—Skeleton of a tortoise with the plastron removed. (a), cervical vertebrae; (b), carapace; (c), vertebrae; (d), ribs; (e), marginal bones of the carapace; (f), pelvis; (g), tibia; (h), fibula; (i), femur; (k), scapula, and (l) acromion. Note how the ribs and vertebrae are fused to the carapace. See Chapter 9 for more information.
The last group of reptiles to consider is the Archosauria (Figure 3.61). Not only do they have the two temporal fenestrae, but they also have an additional anteorbital fenestra (“before eye window”) located rostrally (toward the nose) from the orbit (eye socket) and a mandibular fenestra on the lower jaw (Figure 3.62). In addition, they have a thecodont dentition, with the teeth in sockets. The archosaurs include the crocodylians, pterosaurs, nonavian dinosaurs, and birds—in other words, a wildly diverse group of vertebrates! Archosaurs first appeared in the fossil record in the Permian period and greatly diversified in the Late Triassic period, remaining the dominant vertebrate for the Jurassic and Cretaceous periods.

Figure 3.61—A variety of extant and extinct archosaurs. Left column, top to bottom: mallard ducks Anas platyrhynchos, the pterosaur Dimorphodon macronyx, the rauisuchian Saurosuchus galilei, and the theropod dinosaur Deinonychus. Right column: Tyrannosaurus rex, Triceratops horridus, and the Nile crocodile Crocodylus niloticus (with fellow archosaurs in the background!).

Figure 3.62—An archosaur showing the various openings of the skull. Temporal fenestrae are in brown (right of image), the orbit is green (with sclerotic ring inside of it), the nostril is green (left of image), and the anteorbital fenestra (between orbit and nostril) and mandibular fenestra (on lower jaw) are blue.
Archosaurs are divided into stem-archosaurs plus two groups: the Pseudosuchia (sometimes called the Crurotarsi) and Avemetatarsalia (Figure 3.63). The Pseudosuchia (“false crocodile”) include the Crocodylomorpha and a host of extinct relatives, including aetosaurs, ornithosuchids, and rauisuchians (Figure 3.64). The extant crocodylomorphs have 27 species, including crocodylians, alligators, and gharials. Extant crocodylomorphs are generally large, semiaquatic carnivores. While the crocs might be our go-to image for the Pseudosuchia, the extinct pseudosuchians were wildly diverse, including both bipedal and quadrupedal herbivores, giant carnivores, heavily armored forms, and even some toothless forms.

Figure 3.63—Phylogenetic tree of the Archosauria, modified from Lee et al., 2020.

Figure 3.64—Various pseudosuchians. First four rows, clockwise from top right: Gavialis gangeticus, an extant crocodilian; Saurosuchus galilei, a rauisuchian from the Late Triassic of Argentina; Litargosuchus leptorhynchus, crocodylomorph from the Early Jurassic of South Africa; Chenanisuchus lateroculi, a dyrosaurid crocodylomorph from the Late Paleocene of Morocco; Dakosaurus maximus from the Late Jurassic of Western Europe; Longosuchus meani, an aetosaur from the Late Triassic of North America. Bottom image: A reconstruction of the Triassic Postosuchus kirkpatricki with a human for scale.
The Pseudosuchia lineage and Avemetatarsalia lineage split from each other approximately 250 million years ago and are largely differentiated by their different ankle structures (Figure 3.65). The Avemetatarsalia include the Pterosauria (flying reptiles like Pteranodon) and the Dinosauria (nonavian dinosaurs and birds).

Figure 3.65—Left: a typical crurotarsal ankle found in Pseudosuchia. Right: a typical mesotarsal ankle found in Avemetatarsalia. T = tibia, F = fibula, A = astragalus, C = calcaneum.
The pterosaurs last shared a common ancestor with the dinosaurs about 216 million years ago and lasted until the end of the Cretaceous period (Figure 3.66). Pterosaurs were the first vertebrate group to achieve active, flapping flight and have a unique wing anatomy compared to birds and bats. This is particularly impressive when one considers that the largest pterosaur, Quetzalcoatlus, weighed more than 70 kg and had wingspans of 12 meters! Even though their basic body plans are quite similar, pterosaurs likely had a variety of feeding modes that overlap with several groups of modern birds, such as insectivory, piscivory, carnivory, and filter feeding. However, this is an area of active study as technological advances have created new possibilities for studying pterosaur diets and feeding mechanics.

Figure 3.66—Examples of Pterosauria. Top left: Anurognathus ammoni; Top right: cast of Rhamphorhynchus muensteri; Bottom left: Wingspan estimates of Dearc sgiathanach; Bottom right: Size estimate of Simurghia robusta.
The Dinosauria historically have been divided into two groups, the Saurischia (“lizard-hipped”) and Ornithischia (“bird-hipped”; Figure 3.67). As the names imply, the two groups have different pelvic girdle arrangements. Saurischians have a pubis that points anteriorly, while ornithischians have a pubis that points posteriorly.

Figure 3.67—Dinosaur hip structure. For both images, anterior is to the left of the image. Left: ornithischian hip; Right: saurischian hip.
The ornithischian dinosaurs include primarily herbivorous dinosaurs, including the ceratopsians (e.g., Triceratops), ankylosaurs, pachycephalosaurs, hadrosaurs (sometimes called “duck-billed dinosaurs”), and stegosaurs (Figure 3.68). Note that despite the name, the birds are not included in the Ornithischia, and their current hip arrangement is a derived feature!

Figure 3.68—Ornithischian dinosaurs. Clockwise, starting with the top left: Heterodontosaurus, Nipponosaurus, Borealopelta, Triceratops, Stegoceras, Stegosaurus.
The saurischian dinosaurs also include some herbivores, such as the Sauropodomorpha (e.g., Diplodocus, Brontosaurus; Figure 3.69), as well as the bipedal, mostly carnivorous Theropoda (e.g., Allosaurus, Tyrannosaurus, Velociraptor; Figure 3.70) and the Aves (birds, nested within the Theropoda).

Figure 3.69—A variety of sauropodomorph dinosaurs. Clockwise from upper left: Eoraptor lunensis, Plateosaurus engelhardti, Brontosaurus excelsus, Europasaurus holgeri, Mamenchisaurus hochuanensis, Nigersaurus taqueti, Argentinosaurus huinculensis, Diplodocus carnegii, Brachiosaurus altithorax.

Figure 3.70—Theropod dinosaurs. Top left: Velociraptor; Top right: Allosaurus fragilis; Bottom: size estimates for two species of Compsognathus.
Ideally, every clade includes a common ancestor and all their descendants sharing synapomorphies. We call this a monophyletic grouping. In 2017, a study found evidence that the Saurischia is not a monophyletic clade, although the Sauropodomorpha and Theropoda both are individually. Since then, paleontologists and evolutionary biologists have found support for several different distributions of these three groups on phylogenetic trees and are still working on sorting out these relationships (Figure 3.71).

Figure 3.71—Three equally plausible phylogenetic trees illustrating the relationships between theropods, ornithischians, and sauropodomorphs.
That being said, the fact that birds (Aves) evolved from a line of theropod dinosaurs is not under dispute. While the most famous fossil showing both dinosaur and avian features may be Archaeopteryx, there is a suite of outstanding feathered dinosaur fossils from China that have further supported the dinosaur-bird hypothesis (Figures 3.72 and 3.73). There are several lines of evidence for this hypothesis, including similarities in integumentary structures such as particular feather structures, various skeletal features (e.g., a keeled sternum, wishbones), egg roosting behaviors, and egg structure. The first definitive bird fossils, about the size of a chicken, appear in rocks that are somewhere in the Middle or Late Jurassic period. It is difficult to point to a particular fossil as being the first bird, as the transition from dinosaur to bird was quite gradual. The evolution of flight is complex and does not just belong to the Aves. Based on what we currently know, wings and the types of asymmetrical feathers that are necessary for flight show up in some of the paravian dinosaurs (the dromaeosaurs—Velociraptor is one of these—and another similar-looking group called troodontids; Figures 3.72) and were likely for display or other nonflight activities. We also know that those same paravian dinosaurs evolved the ability for gliding flight independently of the birds. Finally, powered flight, as we see in extant birds, is something that evolved sometime in or after the Early Cretaceous period. Early Aves, such as Archaeopteryx (Figure 3.73) were missing some of the skeletal features that would support flight muscles. Interestingly, some of the other requirements for flight, such as highly derived brains and potentially birdlike lungs, appeared in theropods first.

Figure 3.72—Examples of paravian dinosaurs. Clockwise from top left: Confuciusornis sanctus from the Cretaceous of China, a dromaeosaur fossil, Microraptor gui from the Cretaceous of China, Anchiornis huxleyi from the Jurassic of China, an extant raven, and the troodontid from the Mesozoic era.

Figure 3.73—Top left: Confuciusornis sanctus, from the Lower Cretaceous of China. Top right and bottom: Archaeopteryx.
We do know that birds first diversified in the Early Cretaceous period, filling some niches we see extant birds in, including semiaquatic birds, large generalists, and small tree-dwelling birds. However, the Neornithes (“new birds”), the group our extant birds belong to, did not diversify until after the Cretaceous period (Figure 3.74). Almost every extant neorthine order appears in the 15 million years after the end-Cretaceous mass extinction event. The radiation of the Neornithes is thanks to a number of adaptations, including those related to flight, perching, and brain development. Today, over 10,000 species of extant birds extend across all seven continents, covering a huge range of niches. They range in size from the tiny bee hummingbird at 2.6 grams and 6 cm long to the largest extant bird, the ostrich at up to 2.8 meters tall and over 150 kg. However, the ostrich is not the largest bird of all time—the giant waterfowl Dromornis stirtoni (263 kg) and the elephant bird Aepyornis maximus (406 kg) get those honors (Figure 3.75).

Figure 3.74—A variety of extant birds illustrating different body, beak, and feather morphologies. Top row (row 1): Red-crested turaco Tauraco erythrolophus, shoebill Balaeniceps rex, and white-tailed tropicbird Phaethon lepturus. Row 2: Steller’s sea eagle Haliaeetus pelagicus, gray crowned crane Balearica regulorum, and Indian peafowl Pavo cristatus. Row 3: Common pigeon Columba livia, Anna’s hummingbird Calypte anna, and Atlantic puffin Fratercula arctica. Row 4: Southern cassowary Casuarius casuarius, rainbow lorikeet Trichoglossus moluccanus, American flamingo Phoenicopterus ruber. Row 5: Gentoo penguin Pygoscelis papua, gray heron Ardea cinerea, blue-footed booby Sula nebouxii. Row 6: Bar-throated minla Actinodura strigula, Eurasian eagle-owl Bubo bubo, and keel-billed toucan Rhamphastos sulfuratus.

Figure 3.75—Top: Aepyornis, a dodo, and an extant ostrich. Bottom: Dromornis stirtoni.
3.4 Summary
The vertebrates are characterized by several embryological features, as well as the presence of a well-developed brain, skull, and vertebrae. The vertebrates include a diverse range of extinct and extant organisms that span all ecosystems across the globe.
Application Questions
- What features classify you (a human) as an osteichthyan? A tetrapod? A reptile? A synapsid? A mammal?
- Is it accurate to say that dinosaurs are extinct? Why or why not?
3.5 Further Reading
- Brazeau, Martin D., and Matt Friedman. “The characters of Paleozoic jawed vertebrates.” Zoological Journal of the Linnean Society 170 (2014): 779–821.
- Delsuc, Frédéric, Henner Brinkmann, Daniel Chourrout, and Hervé Philippe. “Tunicates and not cephalochordates are the closest living relatives of vertebrates.” Nature 439 (2006): 965–968.
- Ferrón, Humberto G., and Philip C. J. Donoghue. “Evolutionary analysis of swimming speed in early vertebrates challenges the ‘New Head Hypothesis.’” Communications Biology 5 (2022): 863.
- Fuge, Lauren. “How did the world’s largest pterosaur fly?” Cosmos, December 8, 2021. https://cosmosmagazine.com/history/palaeontology/how-did-the-worlds-largest-pterosaur-fly/#:~:text=Living%2070%20million%20years%20ago,of%20the%20largest%20known%20bird.
- Gans, Carl, and R. Glen Northcutt. “Neural crest and the origin of vertebrates: A new head.” Science 220 (1983): 268–274.
- Long, John A. “The first jaws.” Science 354 (2016): 280–281.
- Lyson, Tyler R., and Gabriel S. Bever. “Origin and evolution of the turtle body plan.” Annual Review of Ecology, Evolution, and Systematics 51 (2020): 143–166.
- Shubin, Neil. Your Inner Fish. New York: Random House, 2008.
- Weinberg, Samantha. A Fish Caught in Time: The Search for the Coelacanth. New York: HarperCollins, 2001.
3.6 References
- Agnolin, Frederico L., Matias J. Motta, Federico B. Egli, Gastón Lo Coco, and Fernando E. Novas. “Paravian phylogeny and the dinosaur-bird transition: An overview.” Frontiers in Earth Science 6 (2019): 423832.
- Ahlberg, Per E. “Follow the footprints and mind the gaps: A new look at the origin of tetrapods.” Earth and Environmental Science Transactions of the Royal Society of Edinburgh 109 (2018): 115–137.
- Anderson, Jason S. “The phylogenetic trunk: Maximal inclusion of taxa with missing data in an analysis of Lepospondyli (Vertebrata, Tetrapoda).” Systematic Biology 50 (2021): 170–193.
- Anderson, Philip S. L., Matt Friedman, and Marcello Ruta. “Late to the table: Diversification of tetrapod mandibular biomechanics lagged behind the evolution of terrestriality.” Integrative and Comparative Biology 53 (2013): 197–208.
- Averianov, Alexander O. “Eutheria (placental mammals).” Encyclopedia of Life Sciences, September 30, 2020. https://doi.org/10.1002/9780470015902.a0029197.
- Baron, Matthew G., David B. Norman, and Paul M. Barrett. “A new hypothesis of dinosaur relationships and early dinosaur evolution.” Nature 543 (2017): 501–506.
- Benton, Michael J. Vertebrate Paleontology, 4th ed. West Sussex: John Wiley & Sons, 2015.
- Bestwick, Jordan, David M. Unwin, Richard J. Bitler, Donald M. Henderson, and Mark A. Purnell. “Pterosaur dietary hypotheses: A review of ideas and approaches.” Biological Reviews 93 (2018): 2021–2048.
- Botha, Jennifer, and Adam Huttenlocker. “Nonmammalian synapsids.” In Vertebrate Skeletal Histology and Paleohistology, edited by Vivian De Buffrénil, Armand De Ricqlès, Louise Zylberberg, and Kevin Padian, 550–563. New York: CRC Press, 2021.
- Brazeau, Martin D., and Matt Friedman. “The characters of Paleozoic jawed vertebrates.” Zoological Journal of the Linnean Society 170 (2014): 779–821.
- Briscoe, Steven D., and Cliffton W. Ragsdale. “Evolution of the chordate telencephalon.” Current Biology 29 (2019): R647–R662.
- Brusatte, Stephen L., Michael J. Benton, Graeme T. Lloyd, Marcello Ruta, and Steve C. Wang. “Macroevolutionary patterns in the evolutionary radiation of archosaurs (Tetrapoda: Diapsida).” Earth and Environmental Science Transactions of the Royal Society of Edinburgh 101 (2010): 367–382.
- Brusatte, Stephen L., Jingmai K. O’Conner, and Erich D. Jarvis. “The origin and diversification of birds.” Current Biology 25 (2015): PR888–PR898.
- Černý, David, and Ashley L. Simonoff. “Statistical evaluation of character support reveals the instability of higher-level dinosaur phylogeny.” Scientific Reports 13 (2023): 9273.
- Chiappe, Luis M., and Gareth J. Dyke. “The beginnings of birds: Recent discoveries, ongoing arguments, and new directions.” In Major Transitions in Vertebrate Evolution, edited by Jason S. Anderson and Hans-Dieter Sues, 303–306. Bloomington: Indiana University Press, 2007.
- Cifelli, Richard L., and Brian M. Davis. “Marsupial origins.” Science 302 (2003): 1899–1900.
- Ciu, Xindong, Matt Friedman, Tuo Qiao, Yilun Yu, and Min Zhu. “The rapid evolution of lungfish durophagy.” Nature Communications 13 (2022): 2390.
- Coates, Michael I., John A. Finarelli, Ivan J. Sansom, Plamen S. Andreev, Katherine E. Criswell, Kirsten Tietjen, Mark L. Rivers, and Patrick J. La Riviere. “An early chondrichthyan and the evolutionary assembly of a shark body plan.” Proceedings of the Royal Society B 285 (2018): 20172418.
- Coates, Michael I., Marcello Ruta, and Matt Friedman. “Ever since Owen: Changing perspectives on the early evolution of tetrapods.” Annual Review of Ecology, Evolution, and Systematics 39 (2008): 571–592.
- Crouch, Nicholas M. A., and Julia A. Clarke. “Body size evolution in paleognath birds is consistent with Neogene cooling-linked gigantism.” Palaeogeography, Palaeoclimatology, Palaeoecology 532 (2019): 109224.
- Dean, Mason N., and Adam P. Summers. “Mineralized cartilage in the skeleton of chondrichthyan fishes.” Zoology 109 (2006): 164–168.
- DeBiasse, Melissa B., William N. Colgan, Lincoln Harris, Bradley Davidson, and Joseph F. Ryan. “Inferring tunicate relationships and the evolution of the tunicate Hox cluster within the genome of Corella inflata.” Genome Biology and Evolution 12 (2020): 948–964.
- Delarbre, Christiane, Cyrill Gallut, Veronique Barriel, Philippe Janvier, and Gabriel Gachelin. “Complete mitochondrial DNA of the hagfish, Eptatretus burgeri: The comparative analysis of mitochondrial DNA sequence strongly supports the cyclostome monophyly.” Molecular Phylogenetics and Evolution 22 (2002): 184–192.
- DeMar, David G., Jr., Marc E. H. Jones, and Matthew T. Carrano. “A nearly complete skeleton of a new eusphenodontian from the Upper Jurassic Morrison Formation, Wyoming, USA, provides insight into the evolution and diversity of Rhynchocephalia (Reptilia: Lepidosauria).” Journal of Systematic Palaeontology 20 (2022): 1–64.
- Donoghue, Philip C. J., Peter L. Foray, and Richard J. Aldridge. “Conodont affinity and chordate phylogeny.” Biological Reviews 75 (2000): 191–251.
- Donogue, Philip C. J., Anthony Graham, and Robert N. Kelsh. “The origin and evolution of the neural crest.” BioEssays 30 (2008): 530–541.
- Donoghue, Philip C. J., and Joseph N. Keating. “Early vertebrate evolution.” Palaeontology 57 (2014): 879–893.
- Evans, Thomas M., Philippe Janvier, and Margaret F. Docker. “The evolution of lamprey (Petromyzontida) life history and the origin of metamorphosis.” Reviews in Fish Biology and Fisheries 28 (2018): 825–838.
- Flannery, Timothy F., Thomas H. Rich, Patricia Vickers-Rich, Tim Ziegler, E. Grace Veatch, and Kristofer M. Helgen. “A review of monotreme (Monotremata) evolution.” Alcheringa 46 (2022): 3–20.
- Ford, David P., and Roger B. J. Benson. “The phylogeny of early amniotes and the affinities of Parareptilia and Varanopidae.” Nature Ecology and Evolution 4 (2020): 57–65.
- Fortuny, J., J. Marcé-Nogué, S. De Esteban-Trivigno, L. Gil, and A. Galobart. “Temnospondyli bite club: Ecomorphological patterns of the most diverse group of early tetrapods.” Journal of Evolutionary Biology 24 (2011): 2040–2054.
- Friedman, Matt, Michael I. Coates, and Philip Anderson. “First discovery of a primitive coelacanth fin fills a major gap in the evolution of lobed fins and limbs.” Evolution and Development 9 (2007): 329–337.
- Fudge, Douglas S., Sarah Schornom and Shannon Ferraro. “Physiology, biomechanics, and biomimetics of hagfish slime.” Annual Reviews in Biochemistry 84 (2015): 6.1–6.21.
- Gans, Carl, and R. Glen Northcutt. “Neural crest and the origin of vertebrates: A new head.” Science 220 (1983): 268–274.
- Gill, Pamela G., Mark A. Purnell, Nick Crumpton, Kate Robson Brown, Neil J. Gostlin, M. Stampanoni, and Emily J. Rayfield. 2014. “Dietary specializations and diversity in feeding ecology of the earliest stem mammals.” Nature 512 (2014): 303–305.
- Glover, Chris N., Carol Bucking, and Chris M. Wood. “Adaptations to in situ feeding: Novel nutrient acquisition pathways in an ancient vertebrate.” Proceedings of the Royal Society B 278 (2011): 3096–3101.
- Graham, Anthony, and Sebastian M. Shmeld. “The origin and evolution of the ectodermal placodes.” Journal of Anatomy 222 (2013): 32–40.
- Green, Stephen A., Marcos Simoes-Costa, and Marianne E. Bronner. “Evolution of vertebrates as viewed from the crest.” Nature 520 (2015): 474–482.
- Handley, Warren D., Anusuya Chinsamy, Adam M. Yates, and Trevor H. Worthy. “Sexual dimorphism in the late Miocene mihirung Dromornis stirtoni (Aves: Dromornithidae) from the Alcoota Local Fauna of central Australia.” Journal of Vertebrate Paleontology 36 (2016): e1180298.
- Heimberg, Alysha M., Richard Cowper-Sal-lari, Marie Semon, Philip C. J. Donoghue, and Kevin J. Peterson. “microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate.” Proceedings of the National Academy of Sciences U.S.A. 107 (2010): 19379–19383.
- Huttenlocker, Adam K., and Christian A. Sidor. “A basal nonmammaliaform cynodont from the Permian of Zambia and the origins of mammalian endocranial and postcranial anatomy.” Journal of Vertebrate Paleontology 40 (2020): e1827413.
- Janvier, Philippe. “Ostracoderms and the shaping of the gnathostome characters.” In Major Events in Early Vertebrate Evolution: Palaeontology, Phylogeny, Genetics and Development, edited by Per E. Ahlberg, 172–186. London: Taylor and Francis, 2001.
- Johnanson, Zerina. “Vertebrate cranial evolution: Contributions and conflict from the fossil record.” Evolution and Development 25 (2023): 119–133.
- Keating, Joseph N., Chloe L. Marquart, and Philip C. J. Donoghue. “Histology of the heterostracan dermal skeleton: Insight into the origin of the vertebrate mineralised skeleton.” Journal of Morphology 276 (2015): 657–680.
- Kemp, Tom S. “The origin and radiation of therapsids.” In: Chinsamy-Turan, A., Ed. In Forerunners of Mammals: Radiation, Histology, Biology, edited by Anusuya Chinsamy-Turan, 3–28. Bloomington: Indiana University Press, 2011.
- LeBlanc, A. R. H., M. J. MacDougall, Y. Haridy, D. Scott, and R. R. Reusz. “Caudal autonomy as anti-predatory behaviour in Paleozoic reptiles.” Scientific Reports 8 (2018): 3328.
- Lee, Hiu Wai, Borja Esteve-Altava, and Arhat Abzhanov. “Evolutionary and ontogenetic changes of the anatomical organization and modularity in the skull of archosaurs.” Scientific Reports 10 (2020): 16138.
- Luo, Zhe-Xi, Chong-Xi Yuan, Qing-Jin Meng, and Qiang Ji. “A Jurassic eutherian mammal and divergence of marsupials and placentals.” Nature 476 (2011): 442–445.
- Lyson, Tyler R., and Gabriel S. Bever. “Origin and evolution of the turtle body plan.” Annual Review of Ecology, Evolution, and Systematics 51 (2020): 143–166.
- MacDougall Mark J., Neil Brocklehurst, and Jörg Fröbisch. “Species richness and disparity of parareptiles across the end-Permian mass extinction.” Proceedings of the Royal Society B 286 (2019): 20182572.
- Mahé, Kélig, Bruno Emande, and Marc Herbin. “New scale analyses reveal centenarian African coelacanths.” Current Biology 31 (2021): 3621–3628.
- Mallatt J., and J. Sullivan. “28S and 18S rDNA sequences support the monophyly of lampreys and hagfishes.” Molecular Biology and Evolution 15 (1998): 1706–1718.
- Mammal Diversity Database. “Mammal Diversity Database (Version 1.12.1) [Data set].” Zenodo, January 30, 2024. https://doi.org/10.5281/zenodo.10595931.
- Marjanović, David, and Michel Laurin. “Fossils, molecules, divergence times, and the origin of lissamphibians.” Systematic Biology 56 (2007): 369–388.
- Marjanović, David, and Michel Laurin. “The origin(s) of extant amphibians: A review with emphasis on the ‘lepospondyl hypothesis.’” Geodiversitas 35 (2013): 207–272.
- McClennen, Michael, Julian Jenkins, and Mark Uhen. “Paleobiology Database. Version 1.3. Paleobiology Database. Occurrence dataset.” https://doi.org/10.15468/jfqhiu accessed via GBIF.org on 2024-06-19.
- Méndez-Maldonado, Karla, Guillermo A. Vega-López, Manuel J. Aybar, and Iván Velasco. “Neurogenesis from neural crest cells: Molecular mechanisms in the formation of cranial nerves and ganglia.” Frontiers in Cell and Developmental Biology 8 (2020): https://doi.org/10.3389/fcell.2020.00635.
- Miller, Randall F., Richard Cloutier, and Susan Turner. “The oldest articulated chondrichthyan from the Early Devonian period.” Nature 425 (2023): 501–504.
- Molnar, Julia L., Roi Diogo, John R. Hutchinson, and Stephanie E. Pierce. “Reconstructing pectoral appendicular muscle anatomy in fossil fish and tetrapods over the fins-to-limbs transition.” Biological Reviews 93 (2018): 1077–1107.
- Near, Thomas J. and Christine E. Thacker. “Phylogenetic classification of living and fossil ray-finned fishes (Actinopterygii).” Bulletin of the Peabody Museum of Natural History 65 (2024): 3–302.
- Nesbitt, Sterling J. “The early evolution of archosaurs: Relationships and the origin of major clades.” Bulletin of the American Museum of Natural History 352 (2011): 1–292.
- Northcutt, R. Glen. “The new head hypothesis revisited.” Journal of Experimental Zoology 304B (2005): 274–297.
- Padian, Kevin, James R. Cunningham, Wann Langston, and John Conway. “Functional morphology of Quetzalcoatlus Lawson 1975 (Pterodactyloidea: Azhdarchoidea).” Journal of Vertebrate Paleontology 41 (2021): 218–251.
- Pascual, Rosendo, and Edgardo Ortiz-Jaureguizar. “The Gondwanan and South American episodes: Two major and unrelated moments in the history of the South American mammals.” Journal of Mammal Evolution 14 (2007): 75–137.
- Seligmann, Hervé, Jiří Moravec, and Yehudah L. Werner. “Morphological, functional and evolutionary aspects of tail autotomy and regeneration in the ‘living fossil’ Sphenodon (Reptilia: Rhynchocephalia).” Biological Journal of the Linnean Society 93 (2008): 721–743.
- Siddor, Christian A. “Simplification as a trend in synapsid cranial evolution.” Evolution 55 (2001): 1419–1422.
- Sigurdsen, Trond, and David M. Green. “The origin of modern amphibians: A re-evaluation.” Zoological Journal of the Linnean Society 162 (2011): 457–469.
- Simões, Tiago R., and R. Alexander Pyron. “The squamate tree of life.” Bulletin of the Museum of Comparative Zoology 163 (2021): 47–95.
- Suarez, Catalina, Analia M. Forasiepi, María Judith Babot, Tatsuya Shinmira, Javier Luque, Rubén D. Venegas, Edwin A. Cadena, and Francisco J. Goin. “A sabre-tooth predator from the Neotropics: Cranial morphology of Anachlysictis gracilis Goin, 1997 (Metatheria, Thylacosmilidae), based on new specimens from La Venta (Middle Miocene, Colombia).” Geodiversitas 45 (2023): 497–572.
- Uetz, Peter (editor.) “The Reptile Database.” http://www.reptile-database.org, accessed July 1, 2024.
- Wagner, Gunte P., Chris Amemiya, and Frank Ruddle. “Hox cluster duplications and the opportunity for evolutionary novelties.” Proceedings of the U.S. National Academy of Sciences 100 (2003): 14603–14606.
- Walker, J. D., and Geissman, J. W., compilers. Geologic Time Scale v. 6.0: Geological Society of America, 2022, https://doi.org/10.1130/2022.CTS006C.
- Whitenack, Lisa B., Sora L. Kim, and Elizabeth C. Sibert. “Bridging the gap between chondrichthyan paleobiology and biology.” In The Biology of Sharks and Their Relatives, edited by Jeff C. Carrier, Colin A. Simpfendorfer, Michael R. Heithaus, and Kara E. Yopak, 3rd ed., Vol. 1., 1–30. Boca Raton: CRC Press, 2022.
- Zardoya, Rafael, and Axel Meyer. “On the origin of and phylogenetic relationships among living amphibians.” Proceedings of the U.S. National Academy of Sciences 98 (2001): 7380–7383.
- Zelenkov, Nikita V., Alexander V. Labrov, Dmitry B. Startsev, Innessa A. Vislobokova, and Alexey V. Lopatin. “A giant early Pleistocene bird from eastern Europe: Unexpected component of terrestrial faunas at the time of early Homo arrival.” Journal of Vertebrate Paleontology 39 (2019): e1605521.
- Zhang, Gunagjun. “An evo-devo view on the origin of the backbone: Evolutionary development of the vertebrae.” Integrative and Comparative Biology 49 (2009): 178–186.
- Zhou, Chang-Fu, Ke-Qin Gao, Hongyu Yi, Jinzhuang Xue, Quanguo Li, and Richard C. Fox. “Earliest filter-feeding pterosaur from the Jurassic of China and ecological evolution of Pterodactyloidea.” Royal Society Open Science 4 (2017): 160672.
- Zhu, Min, Per E. Ahlberg, Zhaohui Pan, Youan Zhu, Tuo Qiao, Wenjin Zhao, Liantao Jia, and Jing Lu. “A Silurian maxillate placoderm illuminates jaw evolution.” Science 354 (2016): 334–336.
- Zhu, Min, Wenjin Zhao, Liantao Jia, Jing Lu, Tuo Qiao, and Qingming Qu. “The oldest articulated osteichthyan reveals mosaic gnathostome characters.” Nature 458 (2009): 467–474.
Media Attributions
- Fig3.6_NEW
- NEW_Fig3.39
- Fig3.71_NEW

