18 Central Nervous System
Bill Ryerson
Focus Questions—to Guide Your Reading of This Chapter
- How do the sizes of different regions of the brain relate to a vertebrate’s interactions with their environment?
- The spinal cord, in many ways, reflects the structure and function of the brain. In what ways are they similar? Different?
- Why is surface area so important for a cortex?
- Is brain size an indicator of intelligence?
18.1 Introduction
The brain: The phrase alone carries a significant weight. Unlike many of the other structures we cover in this textbook, there is an incredible amount of outside influence and discussion about the structure and function of the brain. Whole fields outside of biology are focused on the workings of the human brain. To be existential, right now your brain is processing information about…your brain. In this chapter, we will focus primarily on the anatomical, developmental, and functional elements of the brain across vertebrates. However, there will be times when we must consider how the broader world interprets this anatomy. This may include some misinterpretations of brain structure and function (some amusing and some negative) as well as how some of these happenings helped us better understand the brain. As the brain also influences and controls so many other elements, this chapter will also refer to several other chapters in this book. These are not necessary in our exploration of the brain, but they may help you put the structures and their related functions in context. There is a lot of specific terminology when referring to the brain and its myriad structures. Throughout this chapter, look for clues and hints that will provide insight into the terminology, and use them to help you further your understanding of the brain.
18.2 General Structure and Function
The brain and the spinal cord make up the central nervous system (CNS; Figure 18.1). Both structures arise from the dorsal hollow nerve cord that is a defining characteristic of all chordates. The brain forms at the anterior end of the nerve cord, and while it shares many characteristics with the spinal cord, it is unique in its development and structure.
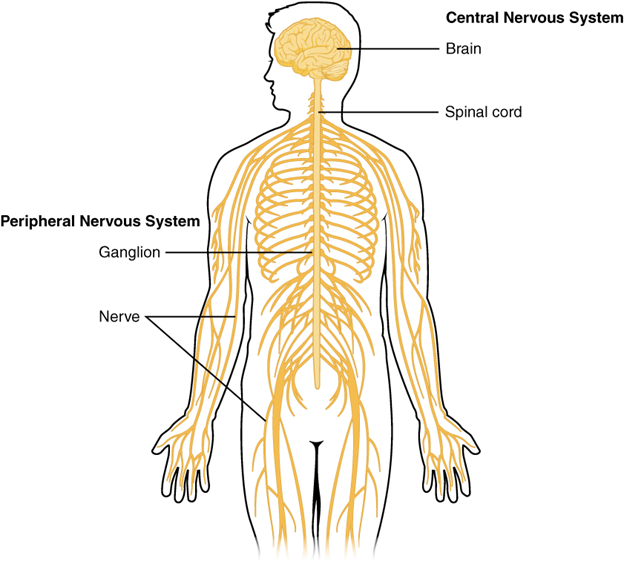
Figure 18.1—The central and peripheral nervous system of a human.
To begin, let us examine the major cellular components of the nervous system. At the cellular level, the brain is predominantly composed of neurons, individual nervous system cells, and neuroglia. Neurons are the cells that make the nervous system work. A generalized neuron illustrates the various pieces of most neurons (Figure 18.2).
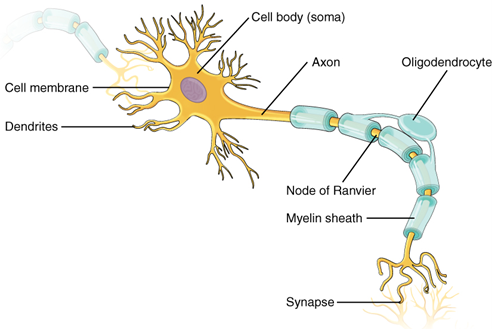
Figure 18.2—A generalized neuron with relevant parts labeled.
Dendrites on neurons are where signals enter the cell, the cell body is the site of integration, and the axons are where nerve signals are sent out. There are many types of neurons, but they all share those same three components. The axons can vary greatly throughout the body and can be extremely long. The longest single axon in the human body runs from the spinal cord to the base of your big toe, almost a full meter in length. Not bad for a cell only a few micrometers in diameter. A key component of a neuron that will help us understand the brain is also found on the axons. In Figure 18.2, note that the axon is covered in a structure called a myelin sheath. The myelin sheath is an insulator and helps the signals move faster down the axon by jumping along gaps in the sheaths known as the Nodes of Ranvier. Myelin sheaths seem to be a vertebrate innovation for increasing the speed of a nerve signal. In invertebrates like the octopus, axons are much larger, the increased diameter helping increase the speed of signal transmission. The myelin-covered axons are quite numerous in the nervous system, their presence able to be observed by the naked eye. In the brain and spinal cord we observe areas that are full of cell bodies and others that make up tracts of axons. The regions full of cell bodies (lacking myelin) appear as darker gray areas in the brain, subsequently called gray matter. The regions with myelin sheaths appear much lighter, and we call those areas white matter (see the spinal cord cross section in Figure 18.3).
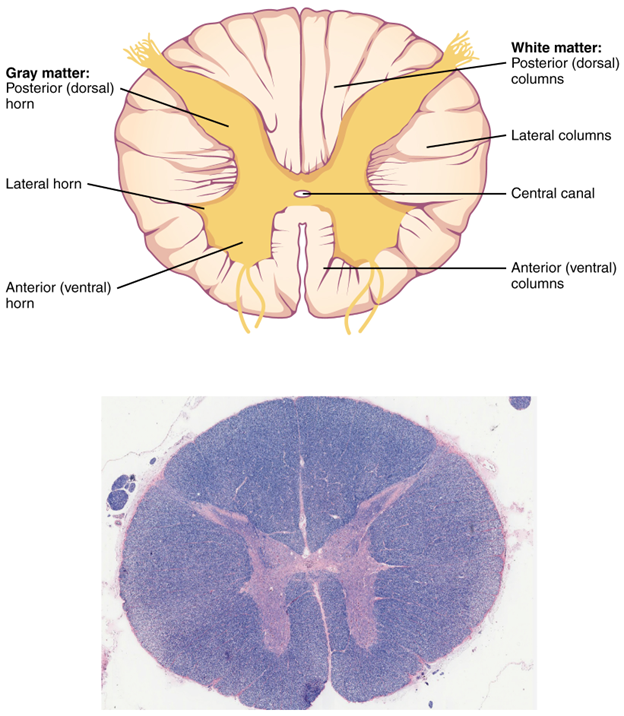
Figure 18.3—Cross section of the spinal cord drawn and via histological section.
This will be one of the ways in which we can differentiate the functions of the different regions of the CNS. If the white matter is full of axons, then we know that these particular regions are responsible for communicating signals from one part of the brain to another, or from the brain to the rest of the body. Gray matter, with the majority of the cell bodies, is the area where incoming and outgoing signals are integrated and processed. As we observe in the spinal cord, the gray matter remains closer to the center of the spinal cord, with the white matter closer to the edges (Figure 18.3). The brain will originally evolve the same way, with the gray matter toward the center and the white matter along the periphery. This distinction will become more important as we explore the evolution of the brain through the vertebrates. While much of our discussion will focus on the nature, location, function, and amount of gray matter in the brain, it would be negligent to ignore the role of the white matter.
While neurons are the majority of the cells in the brain and very important for the function of the nervous system at large, they are not the only cells active in the brain. Neuroglia (or glial cells) are complementary cells that are responsible for monitoring the environment around neurons and responding accordingly. There are many different types of neuroglia: Some function in the immune system response, and others monitor the chemical environment (Figure 18.4).
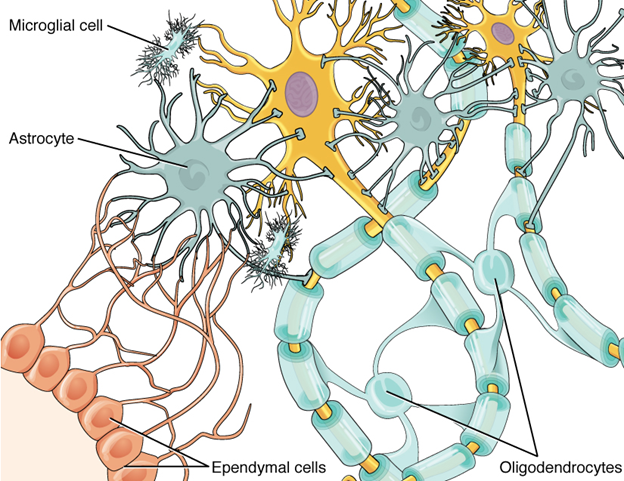
Figure 18.4—Glial cells in the central nervous system.
One type of neuroglia, the astrocytes, uses cellular processes to pull capillaries and neurons closer together, making the movement of oxygen and carbon dioxide more efficient. The oligodendrocytes produce the myelin and wrap it around the axons of the neurons. Ependymal cells line the ventricles of the brain (spaces that form during development) and produce cerebrospinal fluid. There are other glial cells, providing immune system support, regulating the chemical environment, and monitoring the health of neurons.
You will also note in Figure 18.3 an opening at the very center of the spinal cord. Recall from Chapter 2 that one of the chordate characteristics was the presence of a dorsal, hollow nerve cord. While in many vertebrates this space has been filled in quite a bit, the hollow characteristic remains, in the form of a small structure known as the central canal. The central canal is a consequence of the neural tube formation during development (see Chapter 4). Through the central canal passes cerebrospinal fluid (CSF), an essential component of a healthy CNS. The gray matter forms a butterfly-like shape in cross section, and the distinct areas of this butterfly help us separate different regions. Each distinct region is referred to as a horn, and the resulting structures are known as the dorsal and ventral horns. In the dorsal horns, sensory information from sensory receptors is received and processed. In the ventral horns, motor responses leave the spinal cord on their way out to the body through the spinal nerves. Some regions of the spinal cord will also have lateral horns, which are the origin of information for the sympathetic division of the peripheral nervous system (PNS; see Chapter 19).
To understand the general structure of the brain, we have to first examine its structure early on in development. At the earliest stages, the brain can be divided into three regions, anterior to posterior: forebrain, midbrain, and hindbrain (Figure 18.5). Classically, they are also referred to (in order) as the prosencephalon, mesencephalon, and rhombencephalon. From there, the forebrain can be divided into the telencephalon and diencephalon, the midbrain stays the mesencephalon, and the hindbrain divides into the metencephalon and myelencephalon.
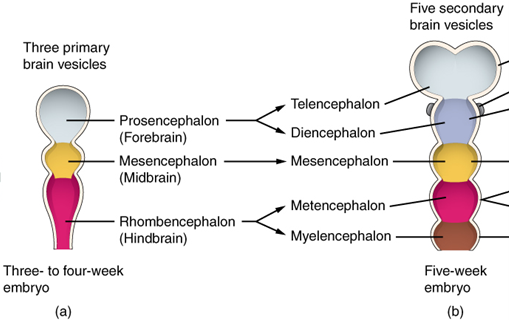
Figure 18.5—The major regions of the brain as they appear in a developing human embryo.
Note that anywhere you see “enceph” or “ceph,” it is referring to the brain or head region. As we progress through the evolutionary sections, we will see that many other structures will be derived from the major regions, and their size/shape has been implicated in better understanding of their function. This will include structures like the cerebellum, derived from the metencephalon of the rhombencephalon/hindbrain. We will introduce new structures as we go, but these are all derived from the previously aforementioned regions. Using the brain of the dogfish shark, Squalus acanthias, as a model, we can explore these concepts (Figure 18.6). The forebrain, midbrain, and hindlimb are color coded and compared with that of a human brain. Note how regions of the brain in the shark appear in a direct line.
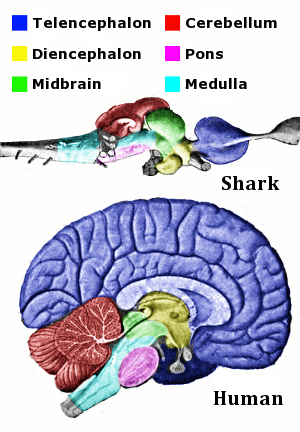
Figure 18.6—The brain of the spiny dogfish, Squalus acanthias, as it compares to that of a human, with anterior being on the right. Homologous regions are color coded for comparison. Brains are not scaled to size.
We will find that mammals are the exception to this rule. There are two structures in Figure 18.6 that have not been introduced yet but are labeled because of their importance to vertebrates: the cerebellum (part of the hindbrain) and the pons (part of the midbrain). The midbrain and hindbrain together form a region that is known as the brain stem. The reasons for calling these regions the brain stem are twofold: (1) all information passing through the brain must go through at least a portion of the brain stem, and (2) many of the functions that are critical for life (heart rate, breathing) are regulated, monitored, and controlled by structures within the brain stem. Damage to the brain stem is likely fatal to most vertebrates.
Three structures that will help us orient ourselves on most brains are the olfactory bulbs, cerebrum, and cerebellum (Figure 18.6; the olfactory bulbs are to the far right on the shark brain and not visible on the human brain). There are often large structures, and changes in their size and shape are useful for understanding changes to the animal itself. Both the olfactory bulbs and cerebrum are involved in the sensory biology of an individual, while the cerebellum helps with the control of movements (more on this in a few sentences). These three structures are useful because they are typically visible from both the lateral and dorsal views across vertebrates and also form a nearly straight line from the anterior/cranial part of the brain to the posterior/caudal. The olfactory bulbs are typically the most anterior of the three. The cerebrum is slightly caudal to the olfactory bulbs, and the cerebellum is the most caudal.
Now that we have set up a brain and oriented ourselves, it is time to introduce a lot of the structures that are common across vertebrates. The most anterior region is the telencephalon, a subdivision of the forebrain/prosencephalon (and host to the olfactory bulbs, the most anterior of the most anterior). The cerebrum is also part of the telencephalon, receiving many different types of sensory input and integrating from multiple senses together. In mammals, the derived cerebrum is also referred to as the cerebral hemispheres, with further differentiation of both structure and function. Posterior to the telencephalon is the diencephalon (still a member of the forebrain). The diencephalon is the home to many structures that direct and regulate many of the body’s “subconscious” functions: heart rate, respiratory rate, hunger, thirst, blood pressure, sleep/wake cycle, body temperature, and more. Additionally, the diencephalon acts as a relay station for signals coming to or from the telencephalon, including motor activation patterns and sensory signals. There are three major structures in the diencephalon: epithalamus, thalamus, and hypothalamus. In addition to the functions already described, it is believed that the thalamus played an important role in the evolution of consciousness, a vertebrate feature that is the subject of intense debate. The hypothalamus is an important connection between the nervous system and the endocrine system, with a physical connection to the pituitary gland. In humans (more on this later), the hypothalamus is the beginning point for the production of the sex hormones testosterone and estrogen (among others). The mesencephalon, or midbrain, also serves as a relay station for many signals originating in different areas. In particular, the midbrain is associated with alertness (in mammals), sleep/wake cycles, relaying visual and auditory information, and body temperature. Last, there is the hindbrain, made up of the metencephalon and myelencephalon. The metencephalon is the host to two major brain structures: cerebellum and pons. The cerebellum is an important gateway from the brain to the rest of the body. The cerebellum is known to be involved in movement or motor patterns. However, it does not generate the motor patterns involved in movement. Instead, what the cerebellum is doing is coordinating the movements in response to other signals that are being sent and received by the brain. This includes the timing and precision of movements. Imagine throwing darts at a dartboard. As you line up your shot, you are receiving visual signals from your eyes about the target. You are also receiving information about the position of your hand relative to the rest of your body. Then you have to coordinate all the muscles involved in the actual throwing of the dart. The cerebellum is playing a large role in coordinating all this information to make sure you hit the bull’s-eye. The pons is home to several cranial nerves (Chapter 19—Peripheral Nervous System) but is also one of the centers of respiration. In humans, the pons plays a role in switching from inhalation to exhalation. The myelencephalon is home to the medulla oblongata. The medulla oblongata is the last major structure in the brain. It has quite a few different functions, but it is mostly associated with regulating elements of heart rate, blood pressure, and respiration. In mammals, it is also known to play a role in sneezing and vomiting. Sneezing and vomiting are good examples of complex behaviors that are involuntary in nature.
With all these elements working together, the brain functions as the control center of the body. Information from the senses is received by the brain, and coordinated responses to stimuli originate from the brain. The brain is also the primary center for the regulation of the other body systems. We will focus on these systems and their regulation primarily in the section on humans to better understand how the different elements of the brain come together. However, that does not mean we cannot glean a few helpful elements now to help elucidate the names, structures, and function of those structures. Large regions of the brain are given one of a few names: cortex, hemisphere, or lobe. These lobes or cortexes are subsequently identified by either location or function. For example, the optic lobe is the location where signals from the eyes are received in the brain, while the parietal lobe is found directly under the parietal bone in humans. Whenever we introduce a new location or structure, take a moment to consider why it has that name and what its function may be.
One last element in this discussion on the general structure of the brain is the fate of the central canal. You may recall the central canal from the general structure of the spinal cord. The central canal is a small channel at the center of the spinal cord that is the direct result of the formation of the neural tube. As the brain grows, turns, and twists during its development (more on this in the next section), the central canal will also get twisted, contorted, and altered as well. Within the brains of vertebrates, the result of this twisting and growing is the formation of ventricles. Ventricles are cavities within the brain that allow for the circulation of cerebrospinal fluid (Figure 18.7). The number of observed ventricles and their location will vary within vertebrates. However, one consistent fact is that all the ventricles are connected to each other and the central canal! This allows the cerebrospinal fluid to circulate throughout the entire CNS.
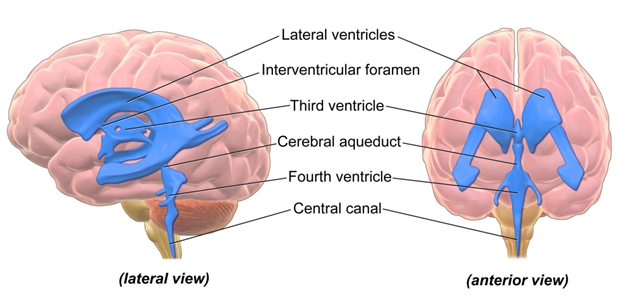
Figure 18.7—A visual representation of the ventricles within the human brain. All the blue regions are open spaces filled with cerebrospinal fluid.
In addition to the physical structure of the brain itself, there are several different levels of protection that are fundamentally part of the structures we must take note of. The skull is the most obvious layer of protection, a bony case that completely surrounds the brain. However, the skull receives its own chapter in this textbook (Chapter 8—The Skull). Between the skull and the brain are several additional layers of protection. Three connective tissue layers form an almost impenetrable barrier around the brain, including preventing the passing of blood and extremely small microbes through their layers (Figure 18.8).
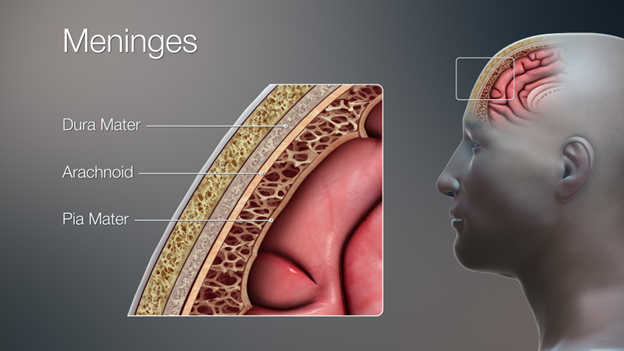
Figure 18.8—The three meninges deep to the skull and superficial to the brain.
These are the meninges (from the Greek word for “membrane”). Each layer of the meninges is known as a “mater” from the Latin word for “mother.” The three meninges are quite different in their structure and their function. The outermost layer is known as the dura mater (“tough mother”). The dura mater is the thickest of the meninges, composed of multiple layers of connective tissue. It serves as the physical barrier between the brain and its outside environment. The middle layer is known as the arachnoid mater, named for a web of filaments that stretch from the arachnoid mater closer to the surface of the brain. Underneath the arachnoid mater, these filaments result in a relatively open space known as the subarachnoid space. Within this space are the large arteries and veins that are the basis of the blood supply for the brain. The brain is an extremely energy-hungry organ, with an incredible supply of blood to provide the nutrients and oxygen required to keep it functioning. The web of filaments help secure the blood vessels and keep them closely associated with the surface of the brain. The last layer, the pia mater, is an ultrathin layer that is in direct contact with the brain. Capillaries pass through the pia mater as they enter and leave the brain.
Along with the meninges, there is a special fluid known as cerebrospinal fluid (CSF) that circulates throughout the brain. Produced by the aforementioned ependymal cells, CSF moves through the open spaces within the brain and in the subarachnoid spaces. CSF provides many support functions for the brain. It helps regulate blood flow via pressure changes. It helps pull wastes as it circulates and also serves as an additional level of immune system protection for the brain (in humans, we sample CSF from the spinal cord to look for infections). Critically, it also acts as a chemical and physical buffer. In the case of trauma, CSF serves as a bit of cushion to prevent potential brain damage. CSF circulates around the brain but also through specialized open regions known as ventricles. Ventricles form during development as the brain grows and folds over itself. They are all interconnected, allowing the CSF to flow through the ventricles to the subarachnoid space and vice versa.
18.3 Development
The brain originates as part of the dorsal hollow nerve cord, one of the characteristics shared by all chordates. The cord itself forms during neurulation (see Chapter 4—Embryology for more on the process of neurulation). The tube remains just that, a tube that terminates at the anterior end of the body. Early on in development, the most anterior portion of the tube begins to enlarge (Figure 18.9).
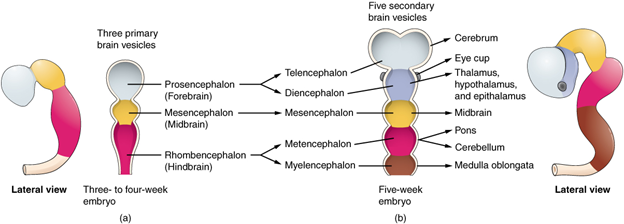
Figure 18.9—The early developmental stages of the human brain.
Three small “bubbles” begin to differentiate themselves, and at this stage we can refer to them as the primary vesicles. The primary vesicles correspond to the three regions of the brain: forebrain (prosencephalon), midbrain (mesencephalon), and hindbrain (rhombencephalon). At this point, it is easy to still think of the neural tube as forming a straight line, but the reality is that the developing brain begins to fold and bend at this stage. Ultimately, in many vertebrates, the brain will resemble a more linear structure, but several of these steps will still occur during development. During the development of the primary vesicles, the brain will “fold” in two locations (both on the ventral portion), then in a third dorsal location. These folds will cause the three primary vesicles to subdivide into the five secondary vesicles: the telencephalon, diencephalon, mesencephalon, metencephalon, and myelencephalon we have already met. We call these folds flexures. The first flexure is the cephalic flexure, occurring in the region of the mesencephalon. Shortly after the appearance of the cephalic flexure, the cervical flexure will appear. The cervical flexure is an especially useful landmark for monitoring the development of the brain, as it occurs near the separation of the hindbrain from the spinal cord. A short time later (2–3 weeks in humans), a third flexure will appear on the dorsal side of the developing brain. This is the pontine flexure, separating the metencephalon from the myelencephalon. A small layer of tissue will remain, creating a roof for the developing fourth ventricle.
Speaking of the ventricles, this is now a good point to reintroduce them and discuss their development. The developing neural tube is hollow at its center. With the formation of the vesicles and future brain regions, the hollow part of the tube does not disappear. Instead, it may get shifted, turned, or twisted around, but the space remains. These will eventually become the ventricles, meaning that every brain region should be associated with at least one ventricle. There is some variation in the naming of the ventricles, so we will be using the human ones as an example for this section. The telencephalon, as it subdivides and grows more laterally, becomes the home of the lateral ventricles. The diencephalon is host to the third ventricle. The cerebral aqueduct passes through the mesencephalon running into the fourth ventricle in the metencephalon and myelencephalon. As we would move through the hindbrain into the spinal cord, the ventricles narrow into the center of the spinal cord, and we refer to it as the central canal. It is important to note that all the ventricles/aqueducts/canals are linked to one another, forming one continuous path that moves throughout the entirety of the brain. Now you might be asking yourself, Why are there no first or second ventricles? These are the lateral ventricles. It is difficult to refer to one or the other as “first,” as they align quite closely from front to back, and they just happen to have moved laterally during development.
After the first few weeks of development, the brain really begins to take its individual shape across the vertebrates. In the interest of space, we will not cover the last developmental stages of all the vertebrates. Instead, we will focus on one group of vertebrates with a fascinating story on brain development, the mammals. The developing mammal brain begins as every other vertebrate brain, following the steps we discussed in Section 18.3, Development. Once the five regions have begun to differentiate and start to shift in place, the different regions begin to expand and grow in size.
For the majority of the vertebrates, the remaining stages of development are rather unextraordinary from a bigger anatomical perspective. At the cellular and genetic level, there are still many things that must divide, migrate, get turned on, and more before the brain can be fully functional. However, there is one special case that we should consider in fuller detail. The mammalian brain undergoes quite the transformation even after the development of the secondary vesicles and five major regions. If we look at our shark brain (Figure 18.6), we can roughly see all the major brain regions directly in a line. Compare this to what you see in the human brain, also in Figure 18.6. From the external viewpoint, you cannot see too many regions of the brain beyond the cerebrum and cerebellum. You can only see the diencephalon and mesencephalon in a midsagittal view or by removing the cerebral hemispheres. At first glance, you may think that these regions have shrunk or been lost in evolutionary time. In reality, it has nothing to do with the diencephalon and mesencephalon at all. The telencephalon in mammals grows fast, much faster than the other brain structures during the early stages of development. In addition to the telencephalon growing faster than the other regions of the brain, the telencephalon also grows faster than the developing skull. This is where the shift occurs. As the growing telencephalon pushes its way forward, it runs into the frontal bones of the skull. However, the telencephalon cannot stop growing. Instead, it pushes upward and then backward. In doing so, it ends up actually wrapping around the diencephalon and mesencephalon. These regions of the brain become almost completely encased by the telencephalon. As we will discuss later, some mammals (like humans) get even more complex. The telencephalon grows so fast that it will also almost fold over itself, with peaks, ridges, and valleys creating an extremely convoluted surface to the cerebrum.
The spinal cord is an extension of the developmental processes that form the brain. Recall the neural tube (Chapter 4) and how differing growth in the anterior end of the neural tube eventually resulted in the formation of the brain. The posterior end of the neural tube becomes the spinal cord. The central canal remains, without all the folding that occurs with the ventricles.
18.4 Evolution of the Spinal Cord
The evolution of the spinal cord is particularly understudied in vertebrates. The most popular hypothesis is that the spinal cord is strongly conserved across the vertebrates, with only minor changes in the larger spinal cord structure across the vertebrate groups. However, this does not mean there are no changes—they are just beyond the scope of this book. For this discussion, we will separate the vertebrates into the fishes and the tetrapods to highlight the major differences.
Fishes
The fishes have a few minor changes in the spinal cord from the general structure highlighted earlier. The spinal cord is present throughout the entirety of the vertebral column from the head to the tail. In the hagfish and lamprey, the spinal cord does not have the distinct gray matter horns described earlier; gray and white matter are distributed throughout the entire spinal cord. Dorsal and ventral gray matter horns can be observed in Chondrichthyes and Osteichthyes. Lateral horns are not found in Chondrichthyes but can be found in the Osteichthyes. There is also a more subtle structural differentiation observed in the bony fishes. Using specialized staining techniques, it is possible to further differentiate the gray matter of the ventral horn into two subregions. The dorsomedial aspect of the ventral horn is most strongly linked with the axial musculature. The ventral aspect is most strongly linked to the pectoral and pelvic fins. As you near the caudal (tail) end of the animal, the spinal cord diminishes in size. Intuitively, this should not come as much of a surprise. Individual nerves branch off the spinal cord, and as you move farther down the animal, there are fewer nerves to branch off. As such, the cord decreases in diameter but remains a solid cord throughout the entirety of its length.
Tetrapods
The spinal cord in vertebrates is observed to have a few more changes from the basal fish condition. The first is that the overall shape and size of the spinal cord changes along its entire length. In the fishes, we noted that the spinal cord continues the length of the vertebral column, with a decrease in size caudally. The spinal cord of tetrapods does not always continue the length of the vertebral column (Figure 18.10).
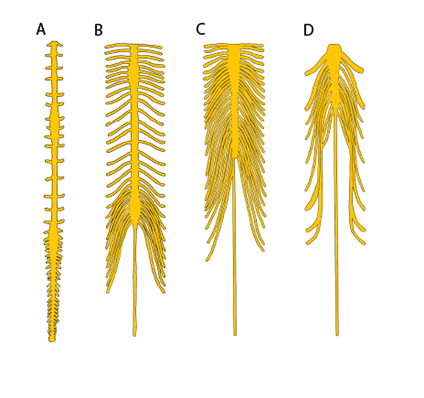
Figure 18.10—The spinal cords of four different tetrapod species: (A) red-eared slider, Pseudemys scripta elegans; (B) harbor seal, Phoca vitulina; (C) human, Homo sapiens; (D) common toad, Bufo bufo.
Some amphibians, reptiles, and birds will have spinal cords that abruptly end prior to the end of the vertebral column, and others may have just a few tracts continue onward. The mammals follow a different trajectory. As we saw in other vertebrates, the spinal cord decreases in diameter in mammals. Moving caudally, however, individual nerve fibers within the spinal cord separate and spread out caudally. Because of its appearance (as though the spinal cord has frayed apart), this area of the spinal cord is known as the cauda equina (Latin, “horse’s tail”) for this reason. This has several clinical implications for veterinary and human medicine (Box 18.1). Note that the cauda equina is still contained within the meninges and still considered part of the spinal cord.
Along the spinal cord in the tetrapods, there are regions where the spinal cord is enlarged. In mammals, these can easily be observed (Figure 18.10B). The cervical enlargement and lumbar enlargement appear as thickenings in the spinal cord. These correspond roughly to where the major spinal nerves (Chapter 19) of the limbs will originate. Within the spinal cord, we can also see changes around these same regions. While the tetrapods retain the horns and gray matter / white matter distinction we observed in the cartilaginous and bony fishes, there is considerable variation among (and within) groups of vertebrates (Figure 18.11). Even within an individual, the shape of the horns will vary along the length of the spinal cord (Figure 18.12). The most supported hypothesis is that these changes coincide with the spinal enlargements and reflect the unique demands of terrestrial locomotion.
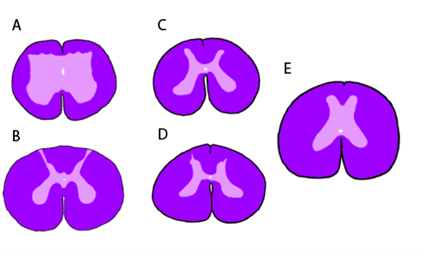
Figure 18.11—Transverse sections of the spinal cord (stained as in Figure 18.3) at the level of the seventh vertebrae in an assortment of amphibian and reptiles: (A) common toad, Bufo bufo; (B) red-eared slider, Pseudemys scripta elegans; (C) Hermann’s tortoise, Testudo hermanni; (D) Argentine black-and-white tegu, Tupinambris merianae; and (E) reticulated python, Malayopython reticulatus.
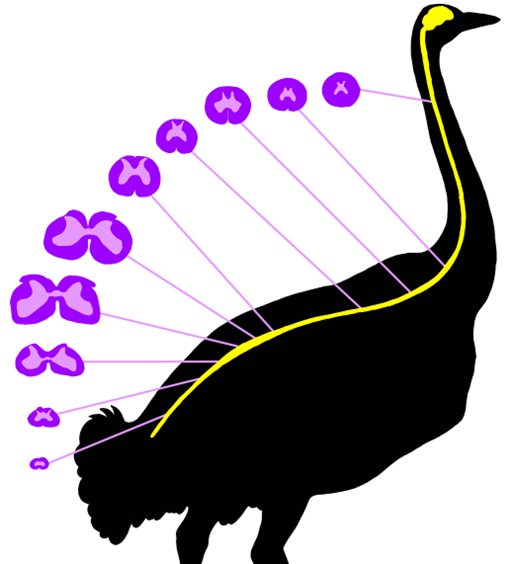
Figure 18.12—Variation of spinal cord structure in an ostrich, redrawn from Streeter, 1904. The spinal cord cross sections have the same color pattern as they would stain histologically. The lighter pink regions are gray matter, and the darker purple ones are white matter.
Box 18.1—A Lumbar Puncture
There are many places where a disease can infiltrate the body. Viruses, bacteria, fungi, and parasites can hide out and damage just about every tissue in the body. However, detecting these disease-causing agents can be difficult if you do not know where to look. Some can be detected (for example) through a blood draw, and for others you can remove and examine a piece of the infected tissue (a biopsy). How would you test for a disease like bacterial meningitis, an infection that causes inflammation of the meninges? You would have to take a sample of the cerebrospinal fluid (CSF) to look for the bacteria responsible. However, sampling the CSF is a dangerous task; you do not want to accidentally puncture the brain or spinal cord. In human medicine, the safest way to sample the CSF is by doing a lumbar puncture (Figure 18.13). In a lumbar puncture, a hypodermic needle is passed into the subdural space between two lumbar vertebrae. As we just discussed, this is the location of the cauda equina. Without the distinct spinal cord, there is more space to safely sample the CSF.
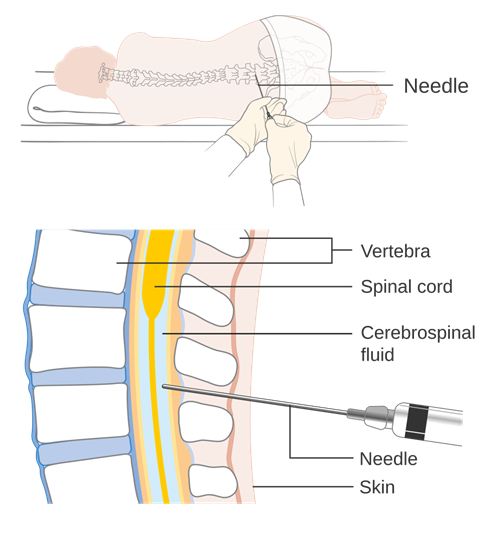
Figure 18.13—A diagram of the appropriate sampling location for a lumbar puncture in humans.
18.5 Evolution of the Brain
Setting the Stage
Before we begin working our way through the evolutionary history of the brain, it is important to understand a little of the interactions between nerves, the senses, and organisms beyond the vertebrates. The neuron is the single unit of the nervous system. The more information that needs to be processed, dealt with, or sent out, the more neurons are necessary to handle that information. However, there is a word of caution to be had. The number of neurons in a brain and/or the size of the brain does not immediately equate with intelligence. Body size plays a large role as well in the size of the brain and number of neurons. The author of this chapter has been fooled by more than one mouse, with his bigger brain and everything.
Prior to the arrival of the vertebrates, an evolutionary novelty appeared that would drastically change how many animals, not just the vertebrates, are structured. In an introductory biology course, you would have learned about many of the characteristics of all animals that helped define lineages like the jellyfish (Cnidaria) from the lobster (Arthropoda). One of the more obvious traits that separates the jellyfish from the lobster is that lobsters are bilaterally symmetrical with a clear left and right side. Vertebrates are bilaterally symmetrical (Figure 18.14).

Figure 18.14—A photo of a bald eagle, Haliaeetus leucocephalus. This photo illustrates both the bilateral symmetry of vertebrates as well as some evidence of cephalization.
At the same time we observe bilateral symmetry appearing in animals, another phenomenon known as cephalization appears. Cephalization is the grouping of the major sensory organs in a “head” region, along with the mouth. The majority of animals have an obvious head, with sensory organs and the mouth dominating the region. Referring to Figure 18.14, what senses can you observe on the head of the eagle? What does this mean for the evolution of the brain? The congregation of sensory organs in a relatively small area has consequences for the nervous system. All the senses need neurons to transmit and process their signals. In this small space, neurons come together in a large group to process all this information. Voilà, you have a brain. You can think of a brain as the largest collection of neurons in a body. An individual neuron is just a neuron. A small collection of neuron cell bodies is a ganglion (plural: ganglia). A large collection of cell bodies is a brain. As all vertebrates exhibit cephalization, we only find brains in the head region. The most favorable hypothesis currently is that as senses gain or wane in use, it should not come as a surprise that those regions of the brain will grow or shrink across evolutionary time. The same will be true for the regions of the brain that coordinate movements or “higher-level” functions (emotions, memories, etc.). However, there has been no compelling evidence that contradicts this hypothesis, which we now refer to as Jerison’s principle of proper mass, from Harry Jerison’s groundbreaking textbook, Evolution of the Brain and Intelligence. So we will continue to assume that the size of a particular region of the brain is correlated with its importance to a species’ biology. However, we should also take a second to remind ourselves that all these comparisons are relative to other parts of the brain or to the individual’s body mass.
It is also important to note that there is considerable variation within groups as to the size and structure of brains. As we will frequently note throughout this next section, there is no “one size fits all” when it comes to defining patterns of brain evolution across large groups. We should also note that brain evolution is a rapidly changing field as more researchers seek answers to questions that have so far eluded us. As we begin this exploration, keep these elements in mind.
Cyclostomes
Extant hagfishes and lampreys represent highly specialized forms of vertebrates and not necessarily those of the common ancestor of all vertebrates. This is especially true when we consider the brain. The brain in the hagfish and lamprey is linear (as it is in most nonmammalian vertebrates). The hagfish brain is highly reduced with few surface distinctions that enable us to identify key structures. The forebrain, midbrain, and hindbrain are observable, and there are ventricles throughout. An olfactory bulb and cerebrum are present. The olfactory bulb is very large relative to the rest of the forebrain, indicating the increased importance of the olfactory system relative to the other senses. It should not be surprising, given the lack of eyes, that the visual processing centers are highly reduced in hagfishes. However, beyond that, there is little to distinguish the hagfish brain from other vertebrates. Many of the structures we identified in our section on generalized vertebrate brains are difficult to locate and identify. The lamprey brain has been well studied for understanding the development of the brain and the changes that can happen over ontogeny, the lifetime of an individual. In many ways, the lamprey brain resembles the developing shark and fish brain (Figure 18.15).
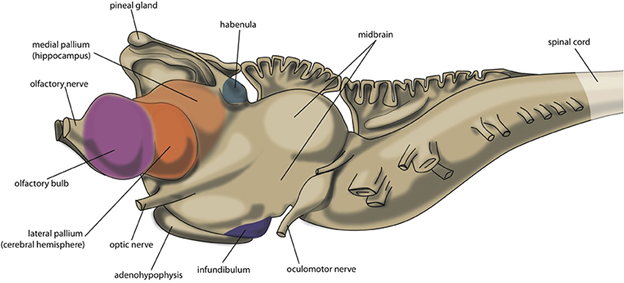
Figure 18.15—The brain of an adult lamprey. The colored regions indicate different areas of the pallium.
One unique element of the brain of the lamprey is the extension of the pineal gland. The pineal gland is a small gland that is part of the diencephalon. In many vertebrates, the pineal gland is associated with sleep-wake cycles. In some vertebrates, including the lamprey, the pineal gland is associated with the parietal eye (sometimes also known as the pineal eye). This eye is covered in more detail in Chapter 20 on the sensory systems. However, for our purposes, a brief summary of its function will help us. The parietal eye is specialized to sense only light or dark. The pineal gland is often closely associated with the parietal eye, as having a light/dark sensor can greatly impact sleep/wake cycles for many vertebrates.
One additional structure that makes its first appearance in the lamprey and will remain through several vertebrate groups are Mauthner neurons. Mauthner neurons are large, individual cells found in the hindbrain. They are the driving force behind the C-start, a characteristic escape response from fishes. In a C-start, the body of a fish bends into roughly a C-shape. It then straightens its body quickly, escaping the situation. Stimulation of Mauthner neurons results in the quick response of muscles to activate in a coordinated pattern. For juvenile lamprey, this allows them to quickly disappear into the sand via activation of their axial musculature to avoid a potential predator.
Chondrichthyes
In most comparative anatomy classes and textbooks, the shark brain serves as the model for the ancestral vertebrate brain. Typically, as we ourselves have done here, that is the brain of the spiny dogfish, Squalus acanthias. By comparison to the lamprey brain, we find that there are several key differences between the two, relating to changes in the sensory and motor systems of the two groups (Figure 18.6). The telencephalon and cerebellum are larger relative to the rest of the brain than we observed in the hagfish. This is suggestive of increased sensory processing and more coordinated muscle movements. The increased size of the cerebellum is the product of increased mobility and control of mobility in the water column. The main side-to-side bending that is characteristic of sharks (and other fishes) is not controlled or relayed through the cerebellum, instead being directed by central pattern generators found within the spinal cord. However, any sharp turns or avoidance behaviors as a response to negative stimuli (an unwanted bump on the snout or contact too close to the eyes) would be smoothed out and coordinated by the cerebellum. Generally, the chondrichthyan brain is elongated and particularly linear, making it easier to identify the major structures of the brain and recognize the changes. However, there is considerable variation among shark brains. With the large diversity of both ecologies and body size in sharks, we can begin to look at how differences within groups shed light on the evolution of the brain and the factors pushing that evolution. Mako sharks and great whites have considerably larger optic tecta (singular: optic tectum; also sometimes referred to as optic lobes) than the dogfish; the Greenland shark and other deep-water species have much smaller visual processing centers. Generally, as body size increases, so too does brain size, which is why we try to use other measures to understand changes to the brain over time. In sharks, relative brain mass (brain mass divided by body mass) has been used to try to tease apart differences across species. One might expect that while brain size does increase with respect to body size, the relative size of the brain would remain the same. However, a trend emerges in sharks and rays that will show up independently in the other major vertebrate lineages as well. Some lineages within groups will develop brains that are much larger relative to body mass and others that are much smaller. This includes changes that might be considered ontogenetic or incorrectly comparing the juvenile of one species to the adult of another. In the sharks, the Carcharhiniformes (tiger sharks, bull sharks, lemon sharks, and more) and Lamniformes (makos, great whites, salmon sharks, and others) share a larger brain-to-body-mass ratio than the other groups. The sharks closely related to the dogfish (squalomorphs) seemingly have smaller relative brains. However, even within the larger groups are species that buck the trend. Among the largest sharks, the basking shark (one of the Lamniformes) has a noticeably smaller relative brain mass than the other members of its group. This provides us with an opportunity to hypothesize why that might be. In the rays, it is the myliobatids (the stingrays and eagle/manta rays) that possess much larger relative brain masses. The skates have reduced relative brain mass.
It is also in the sharks where we first start to see a new type of structure we call a cortex. Earlier in this chapter, we discussed the distinction between gray matter and white matter. Aside from their functional and structural differences, there was also a key difference in where they could be found in the brain. At the center or within the edges of the brain are areas of gray matter called nuclei. Surrounding the nuclei are tracts of white matter. This is true for most areas of the brain and differs across different vertebrate groups. Some sharks, like the great white shark, have an additional layer of gray matter around the outer edge of the cerebellum, known as the cerebellar cortex (Figure 18.16). Additional gray matter means additional cell bodies, which we know provide additional ability to process and integrate information. Our highly active, fast-moving sharks likely need this cortex to help coordinate the tight turns and quick movements in pursuit of prey or evasion of large predators.
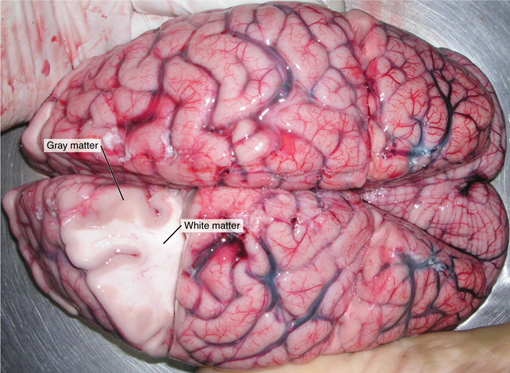
Figure 18.16—The cerebral cortex of the human brain with the additional layer of gray matter on the external surface (easier to visualize than the aforementioned great white). Note that the location of the white matter and gray matter here is reversed from the general vertebrate condition.
Osteichthyes
Given the huge diversity of bony fishes, in their habits, habitats, and more, it is difficult to tease apart general trends in the evolution of the brain. To begin, let us consider how the bony fish move. Before, we stated that the cerebellum helps smooth complex movements. Lineages that use significant body bending in their swimming, like the eels and sturgeons, have a relatively smaller cerebellum compared to other lineages that use their pectoral, pelvic, and caudal fins independently for movement (e.g., freshwater sunfish). Overall, the relative brain mass in bony fishes is larger than the chondrichthyan fishes. Additionally, the size of the optic tectum and telencephalon as a whole is larger in the bony fishes than in the chondrichthyan fishes. Similarly to the chondrichthyan fishes, some lineages have evolved relatively larger brain sizes.
Another interesting pattern that is first observed in the bony fishes is that from a phylogenetic perspective, larger lineages have smaller relative brain mass than smaller lineages. We should stress here that the range of body masses is across a much larger span than that of the chondrichthyan fishes. While the largest fish in the world are sharks, the smallest shark in the world is still 20 cm in length (the dwarf lanternshark). However, there are many bony fish smaller than that size as adults (e.g., the dwarf goby at 7.9 mm) with juveniles only a few millimeters in length at birth. The constraints on brain size do not appear to be impacting the larger lineages. Instead, it is the ultrasmall lineages of bony fish that are pushing the lower limits for how small a functional brain can be. The smallest guppy still needs to see, hear, smell, catch food, evade predators, and find a mate. All these senses, movements, and behaviors require neurons to receive, integrate, and send signals.
One novel structure that we did not discuss in the section on the general structure of the brain is the inferior lobe (Figure 18.17). The inferior lobe of bony fishes occupies the same space as the hypothalamus in the tetrapods and was considered for some time to be equivalent to the hypothalamus. However, there are a few key differences between the inferior lobe and the hypothalamus. Developmentally, there are separate origins for the inferior lobe and the hypothalamus, though there are several shared cell types between the two.
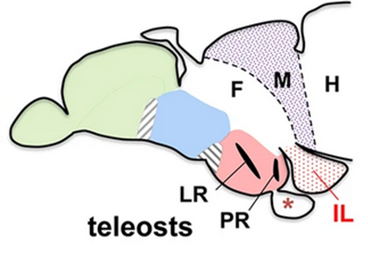
Figure 18.17—A lateral view (anterior to the left) of the brain of a zebrafish, Danio rerio. Green is the telencephalon, blue is the thalamus, and red is the hypothalamus. F—forebrain, M—midbrain, H—hindbrain, LR—lateral recess of hypothalamus, PR—posterior recess, IL—inferior lobe.
Functionally, the inferior lobe seems to be involved in multimodal signal integration. Multimodal signal integration is where a part or parts of the brain take information from different senses and use them together to more completely assess the situation. For example, the sense of smell would be informative for the presence or absence of food, but add in the physical sensations from the lateral line column and you may be able to pinpoint the exact location of the food.
In addition to these other structures, we have to define a new section of the cerebrum. The cerebrum, part of the telencephalon, can be subdivided into even further sections. Wrapping around the ventricle, we can refer to individual segments as pallium. This is specific to the upper layers of the cerebrum. The pallium can be broken further into dorsal pallium, medial pallium, subpallium, and so on (Figure 18.18). For now, the important distinction is that there is going to be a slight change in the structure of the pallium. Note in Figure 18.18 that the structure of the pallium follows our general principle for layering of gray matter and white matter. Gray matter (cell bodies) is found at the center with the white matter tracts (myelinated axons) around the outside of the gray matter. Beginning in the bony fishes and continuing onward, we will find select areas of the pallium where there will be additional gray matter on the outside edge.
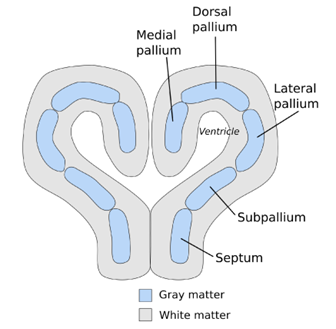
Figure 18.18—A cross section of the forebrain of a teleost fish, highlighting the subdivisions of the gray matter.
What is the advantage of the additional gray matter? More cell bodies means more ability to process incoming information and direct responses. We will find that this is a recurring theme throughout the rest of the vertebrates. When space starts to become limited, where can we add neurons to increase the ability to process and integrate information? The pallium allows for additional neurons with a minimal increase in the overall size of the brain.
Amphibia
The transition to land produced evolutionary novelty across the body for tetrapods, but in the case of the brain, the impacts of the transition are unclear. As we will soon see, this may be because our models for the transition, the extant amphibians, are highly specialized for their lifestyles. Instead, we have relied on endocasts of the braincase in the “fishapods” (like Tiktaalik) and stem tetrapods to shed some light on the changes, but these data are currently sparse.
In extant amphibians, there are several broad trends and interesting differences between the different amphibian groups. Across the fishes, we found that at the phylogenetic scale, brain size relative to body mass increased in several lineages. In the amphibians, we find the opposite. Across the frogs and salamanders, there is a conspicuous reduction in the relative brain size. The functional/evolutionary reasons for this reduction in size are still unclear. One of the most striking reductions is that of the cerebellum in amphibians (Figure 18.19). In the previous section, we noted that as more complex movement behavior evolved in the fishes, the cerebellum increased in size relative to other regions of the brain, a correlation that made intuitive sense given our understanding of the cerebellum’s role in smoothing and coordinating muscle outputs. A much smaller cerebellum would suggest a lack of complexity of movement in the amphibians, but we know that this is not the case. Frogs and salamanders both exhibit a large array of complex locomotor and feeding behaviors, even though the size of their cerebellum is reduced to other parts of the brain when compared with fish. In addition to the relative reduction in the size of the brain, the distinctions between regions are also less apparent in amphibians than in other vertebrate groups.
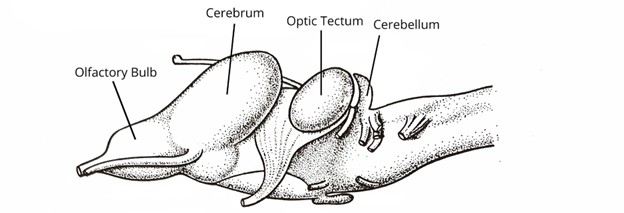
Figure 18.19—A lateral view of the brain of the European grass frog, Rana temporaria.
Despite the general decrease in the relative size of the brain compared to body size across all amphibians from the fishes, there are a few interesting trends that emerge when comparing the different amphibian groups to one another. The anurans, our frogs and toads, have a relatively large optic tectum and increased auditory processing centers. Many frogs are visual hunters and also rely on sound to communicate between individuals of the same species. The salamanders have large olfactory bulbs, and many groups have an enlarged optic tectum as well. This is particularly true for the plethodontid salamanders, in which some species can capture their prey from quite a distance away using their tongue. The increased optic tectum is correlated with their targeting in prey capture.
One final item to note on the amphibian brain: The telencephalon is easily divided into two hemispheres (just like the cerebral hemispheres in humans). Across amphibians, the hemisphere has several different pallial zones. Without getting too far into the weeds, it is important to note that the lateral pallium is quite extensive. It is predominantly associated with olfactory input. Given what we have already discussed with amphibians, it should come as no surprise that these increased areas of gray matter are dedicated to the processing of olfactory information.
Nonavian Reptiles
The reptiles in many ways are quite similar to the amphibians (but larger) and fishes before them. Reptiles have a largely linear brain with a larger cerebellum and telencephalon (Figure 18.20).
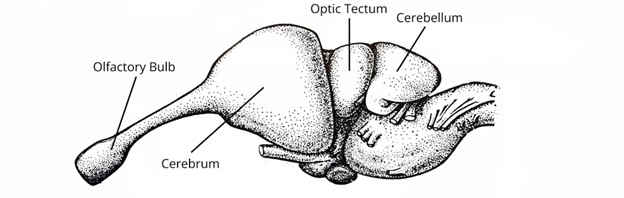
Figure 18.20—Lateral view of the brain of an American alligator, Alligator mississippiensis.
However, a new structure can be observed, one that will change how we examine brains for the remaining vertebrate groups and in general how we as humans view the evolution of intelligence. In the section on chondrichthyan fishes, we introduced the cerebellar cortex, an additional layer of gray matter on the cerebellum that we hypothesize allows for greater maneuverability in the fishes. However, we will observe that a new region of the brain gains a layer of gray matter in reptiles: specifically, the neocortex, a layer of gray matter on the surface of the cerebrum (a part of the telencephalon), which is also referred to as a cerebral cortex. This is considered a distinct separate structure from the pallium we briefly discussed in the fishes and amphibians (though there is still some debate in this regard). Most in the field would consider the cortices to have been derived from the pallium. All parts of the neocortex are part of the pallium, but not all parts of the pallium are part of the neocortex. The cerebral cortex in reptiles is structurally very similar to what we will observe in mammals, although all evidence points to it having arisen independently. The reptilian cortex has many fewer subdivisions than the mammalian one, only a medial, lateral, and dorsal region. The function of the lateral cortex is primarily olfactory, while the dorsal cortex receives some visual information.
The simplified function of the cerebral cortex is that it greatly increases the number of cell bodies available in the cerebrum for the processing of incoming sensory information and the generation of motor outputs. In particular, it would seem that the new cerebral cortex allows for the processing of multimodal information. Multimodal integration means using two distinct pieces of information and putting them together to solve a problem or provide context. Migrating sea turtles are well known for returning to the exact beach from which they were born. Using a combination of magnetic, visual, and olfactory cues, they can find their way back from thousands of miles away. It also allows for the generation of complex and learned behaviors. Monitor lizards can solve puzzles and snakes can cross large gaps between trees using a combination of sensory information and motor control.
Aves
In our exploration of the evolution of the brain in vertebrates, we found that different regions of the brain increase in size relative to others in response to changes in their sensory systems. As we focus here on birds, the most obvious change is the increased relative size of the telencephalon. The telencephalon is vastly enlarged in comparison to the other regions of the brain. There is also typically a very large optic tectum. There is also a relative increase in the size of the cerebellum compared to other vertebrate groups (Figure 18.21). However, we should begin outright by saying that the birds have some of the most drastic differences in the size of brain regions across species for any vertebrate group, a testament to the many different ecological niches they have been able to fill.
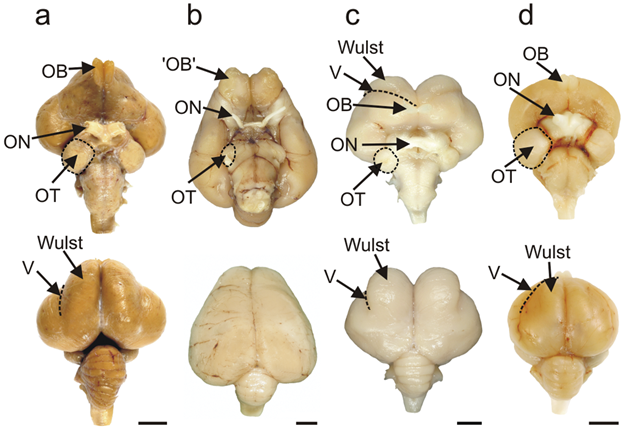
Figure 18.21—Ventral and dorsal views of a few selected bird species. (A) emu; (B) kiwi; (C) barn owl; (D) pigeon. Relevant regions have been labeled: OB—olfactory bulb; ON—optic nerve; OT—optic tectum; V—vallecula (border of the wulst). Anterior is toward the top of the page for each brain.
The most obvious explanation for the increased telencephalon, optic tectum, and cerebellum is that they evolved in response to (or in tandem with) the evolution of powered flight. Similar increases in size of these three regions have also been identified from endocasts of pterosaurs, flying reptiles (not dinosaurs!) that are a distinct branch separate from the bird lineage. Intuitively, this should make sense. Take a quick look at the video of a kestrel hovering over an airfield in the United Kingdom (Kestrel Hovering). This individual bird is hunting for smaller birds and mammals below it. Birds, especially the hawks, falcons, and eagles, are well known for having exceptional vision. Exceptional vision requires exceptional processing power. Flying requires not just a lot of muscular power and energy but the ability to take into account all the information that it takes to remain in the air. For most terrestrial tetrapods, knowing where they are in space is essentially a two-dimensional game. Birds have added a third dimension to this and must maneuver through that dimension accordingly. Changes in wind direction, height, and velocity all require sensory input and changes to be made via wing position and angle. Flight, it seems, has resulted in a very large cerebrum, balancing all the multimodal input that prevents a disastrous crash for the bird.
One thing to note that we have not discussed frequently to this point is how the brain sits within the skull. When we think of the brain in the skull, we probably do not imagine it as an incredibly tight fit but also understand that there is not a lot of extra space within the skull as well. There is a lot of variation (unsurprisingly) in how much space there is within the skull, but the birds make the most of that space. Bird brains are quite large for the brain cavity, leaving only a minimal amount of space between the brain and the inner surface of the skull. There is so little space that birds only have two meninges, and both layers are quite thin.
An interesting twist on brain anatomy in the birds is not so obvious from the surface. Despite not developing the ridges and valleys we will soon see in mammals, the avian brain has one major functional adaptation for increasing its ability to process large amounts of information, and that is by increasing the density of the cells in the cortex.
The bird telencephalon also has one new unique structure. Known as the eminentiae sagittales (or wulsts), these small lobes sit on the dorsal surface of the telencephalon. The wulsts are considered to be an additional area of visual processing for birds. In particular, they are considered essential for giving birds stereoscopic vision, allowing predatory birds to determine depth/distance based purely on vision. What’s most interesting is that the wulsts are a site of considerable variation across bird groups (Figure 18.21). Owls have particularly large wulsts, while those of the kiwi are barely visible at all. This should make sense if we consider this fact in the context of their behavior and feeding ecology. Owls are nocturnal and fly (many species maneuver silently through dense forests), while kiwis rely on mechanosensors in their beaks and don’t fly.
Mammals
Much like we observed in the birds, there are several changes to the brain in mammals that require some investigation. However, in mammals there are quite a few changes that occur that on their surface do not seem as clearly defined as some of the previous changes we discussed. Like birds, the cerebrum is very enlarged, but in addition to the increased size, there are a few structural changes that occur. In the smaller mammals, like mice, there are comparatively fewer differences in the overall brain structure when compared with the brain structure we explored in the birds. However, as mammals get larger, an interesting phenomenon occurs that has wide-reaching impacts on the structure and function of the brain. Compare the mouse brain to that of the human (Figure 18.22).
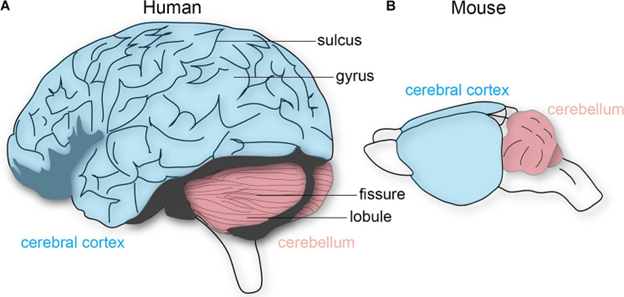
Figure 18.22—Lateral views of a human and mouse brain. Note the “folds” visible on the human brain but not the mouse brain.
Take a closer look at the cerebral cortex in Figure 18.22. There is some size disparity, to be sure, but look more closely at the drawing. You may notice that there are ridges, lines, and grooves along the surface of the cerebral cortex. This is not a human trait but a significant component of many mammal brains, particularly as species get larger (Figure 18.23). The surface of the cerebral cortex is covered in small ridges and grooves. These are known as gyri (singular: gyrus) for the ridges and sulci (singular: sulcus) for the grooves. How they form requires us to take into account challenges in both physics and how brains develop during ontogeny. As we learned in the developmental section of this chapter (Section 18.3), the telencephalon grows fast in mammals, very fast. So fast that it ends up covering most of the diencephalon. However, this growth is not equal across the entirety of the telencephalon. During development, areas of the telencephalon that grow faster end up “pushing aside” other regions. As the differential growth continues, the folds and ridges become the gyri and sulci. Do not forget that some of these regions are also likely to be connected to each other already, further limiting the directions in which the growing brain can move.
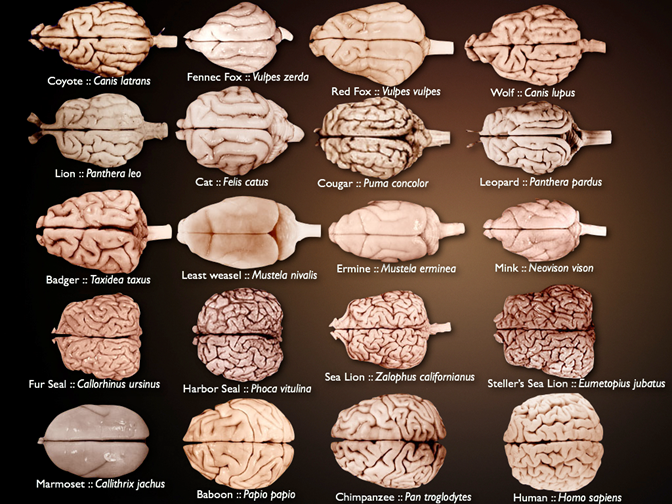
Figure 18.23—Dorsal views of a broad array of mammal brains. Brain size is scaled for the image, not relative size of brain or individual species.
Why is it that we do not have spiky brains, with clear obvious peaks and valleys? Return to our section on development. As the brain continues to grow, the skull continues to act as a physical limit to growth, “flattening” the rising gyri. Sulci can continue to move inward, and we have names for those structures: fissures. Some fissures, like the longitudinal fissure in the primates, are very obvious. The longitudinal fissure is the deep groove that separates what we call the left cerebral hemisphere from the right cerebral hemisphere.
If the gyri and sulci arise from developmental and physical phenomena, do they have functional implications? We know from the reptiles that the cerebral cortex (or neocortex) is defined as more gray matter on the surface of the brain, which results in more cell bodies for the integration and processing of information. The increase in cerebrum size provides more surface for the gray matter. The folds, therefore, are further increases in surface area, allowing for even more gray matter. Unfortunately, this is really the end of our understanding of the functional implications of the folding. As we will shortly see in humans, the cerebral cortex has many functions associated with it, but the question of those functions beyond humans is currently a hotly contested debate.
With all these different regions and all this growth that occurs in mammals, how do these different areas of the brain communicate with each other? To map this out, we will use the human brain as the model, though most of the tracts can be identified in other vertebrates. The first tracts are association fibers. Association fibers connect different parts of the same brain region. For example, looking at the right cerebral hemisphere in humans, fibers that connect the front of the hemisphere with the back would be association fibers. Commissural fibers connect different gray matter brain regions together, almost entirely those involved in the brain that have hemispheres (cerebrum and cerebellum). In humans, there is a structure known as the corpus callosum, a thick band of white matter that is found on the dorsal aspect of the lateral ventricles. The corpus callosum is the largest commissure, allowing communication between the left and right hemispheres of the cerebrum. Finally, there are projection fibers. Projection fibers connect different brain regions from front to back. While commissural fibers might connect different hemispheres, the projection fibers would be what connects the brain stem to the cerebral hemispheres, and vice versa. Projection fibers dissipate as the brain stem becomes the spinal cord and only become known as tracts beyond this point.
Humans
The human brain is arguably the most studied single structure in all of biology and medicine. In most regards, the human brain is quite similar to that of other mammals. However, because of the intensive study that the brain has received over the years, the function of different regions of the brain is more well known in humans than it is in other vertebrates. With this in mind, we can spend more time discussing the function of various elements of the brain.
Broadly, the cerebral hemispheres are associated with the processing of sensory information and the onset of voluntary motor movements. We can break the hemispheres into five lobes: frontal, parietal, temporal, occipital, and insula (Figure 18.24).
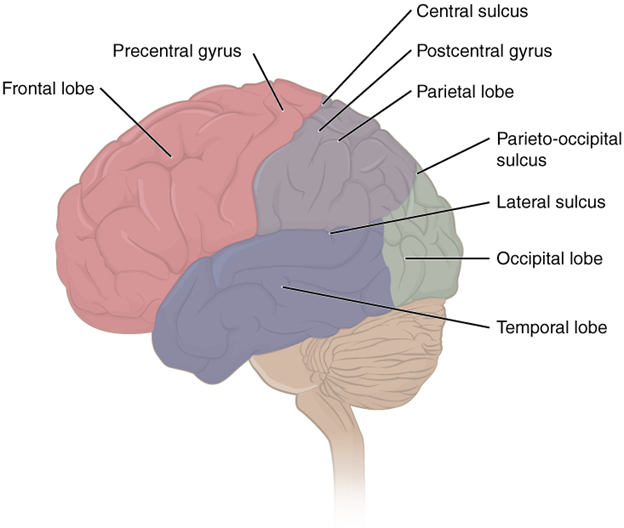
Figure 18.24—The human brain with four of the five lobes of the cerebrum colored in.
Four of the five lobes are visible from the exterior surface of the brain; only the insula is not visible. Pioneering work in 1909 by Korbinian Brodmann, a German neuroscientist, found histological differences all across the cerebral cortex (both within lobes and across lobes), and he used these differences to define more than 50 different regions. Many years later, we determined that those areas correspond quite well to functions within and across the lobes as well. To begin, let us explore the areas that control motor movement across the body. The central sulcus divides the anterior frontal lobe from the parietal lobe that is just posterior to it. The most posterior part of the anterior frontal lobe, just anterior to the central sulcus, is the primary motor cortex (Figure 18.25). You can think of the primary motor cortex as the beginning point for almost all voluntary movement. Just anterior to that is the premotor cortex. The premotor cortex is similar to the primary motor cortex, but it is understood to function predominantly in movements that require considerable practice or planning. Movements or patterns of the movement that you might refer to as “muscle memory” likely are controlled by the premotor cortex. Remember, all these movements are filtered through the cerebellum on their way out, where the different signals for the movements can be coordinated. There are two other regions of motor control, also nearby in the frontal lobe. The first is the frontal eye field, which controls voluntary eye movements. The second is Broca’s area, which coordinates the muscles involved in speech production. It is named for the French physician Pierre Paul Broca, who first discovered that damage in that area resulted in the loss of the ability to speak coherently. The emphasis is on the coordination of the muscles involved in speech. To review, the muscles that contribute to speech include those of the face, tongue, and larynx. While all the muscles may operate just fine in their other tasks, the ability to produce speech (in the form of spoken language) requires significant coordination.
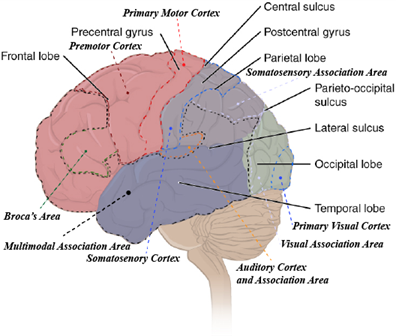
Figure 18.25—The lobes of the brain with dashed lines indicating the cortices and association areas.
Overall, motor cortices are a relatively minor component of the cerebral cortex in humans and restricted to the frontal lobe. The remaining functional regions of the cerebral cortex may span across lobes and can be broken into three components: primary sensory cortices, sensory association cortices, and multimodal association areas (Figure 18.25). The differences between these different regions are quite important. The primary sensory cortices are where the raw signals come into the brain. The association areas are where those raw signals are given context. That context may be a memory or other information. We have all had an experience where someone we knew drastically changed their appearance. When you first saw them, perhaps you did a double-take and needed to verify that it really was them. In this case, the information you were receiving from your primary visual cortex was not matching the context from your visual association area. The same is true for many of the other sensory systems as well. You can recognize particular voices, tastes, smells, and even body position. The sense of touch, housed in the primary somatosensory cortex, is also a key area to identify. It too has an association area, where memories can be informative for textures, shapes, and weight. For example, could you tell the difference between a dry-erase marker and a pencil with your eyes closed? What about a hardcover book and a paperback? Again, memories are providing context to raw signals. So if touch is working similarly to vision and other senses, why take the time to highlight it here? That is because unlike many of the other external senses, your sense of touch occurs across all your skin, but to different sensitivities. Your fingertips are especially sensitive, but the skin over your elbow is much less so. There is a broad correlation between how sensitive touch is in any particular region and how much of the primary somatosensory cortex is devoted to the region. This is often depicted as a 2D representation by something we refer to as the “somatosensory homunculus” (Figure 18.26). Not to be taken literally, as many of the regions overlap with each other, the homunculus nevertheless gives you a great working idea of the regions of the body that are more sensitive to touch.
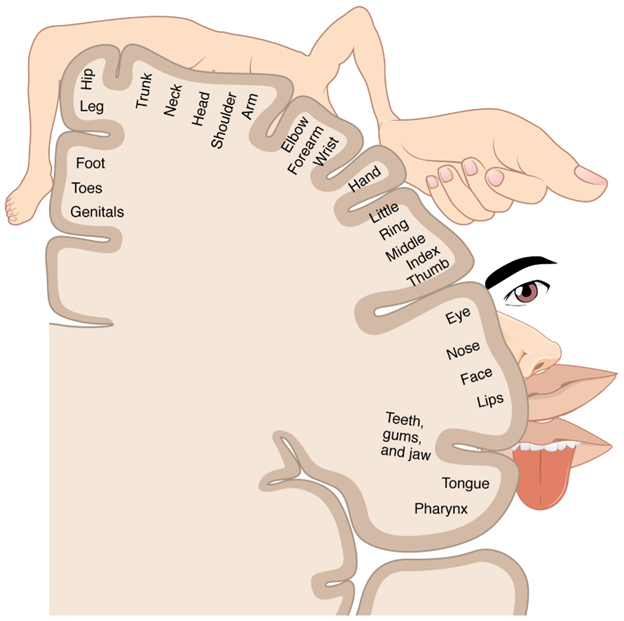
Figure 18.26—The sensory homunculus as it is often depicted in the human brain. The relative size of drawn structures roughly correlates to the amount of area dedicated to interpreting sensory information from that area.
Finally, we come to the multimodal association areas. Their name likely gives away a bit of their function, but it only scratches the surface of what we consider the function of these areas to be. The multimodal association areas are large regions of the cerebral cortex that take different types of information (multimodal) and apply them with memories/context (association) to solve complex problems.
The multimodal association areas can be divided into three major regions: anterior association area, posterior association area, and the limbic system. The anterior association area is arguably the most complex system in the cerebral cortex, from a human perspective. It is the home to almost all of what we might consider our “cognitive” processes: personality, learning, intellect, recall, planning, judgment, and reasoning. In humans, this area is incredibly plastic—that is, it will change based on experience over time. You may have heard the argument of “nature versus nurture” when it comes to positive and negative elements associated with a person’s personality or behavior. This discussion originates from our understanding of the development and function of the anterior association area. This is also the area we associate most closely with the planning and handling of abstract ideas. Complex problems of logistics (I have three errands to run; which order do I do them in?) are handled in the anterior association area. Medically, the anterior association area has attracted the most attention, as damage to that area is correlated with “negative” behaviors and changes to a person’s personality (Box 18.2).
Box 18.2—Phineas Gage, History’s Most Famous (Damaged) Brain
How do we know so much about the regions of the brain that relate to personality? Much of our original understanding stems from a man named Phineas Gage. Phineas Gage was a railroad worker overseeing elements of the construction of a railroad in Vermont in 1848. An accident caused a tamping iron to run through Mr. Gage’s brain. The tamping iron entered his left cheek, just under the zygomatic arch, and passed through his frontal lobe. Amazingly, he not only survived this ordeal but regained consciousness and sat upright in a cart being used to get him to a doctor. He survived the accident, the surgery to remove the tamping iron, and extensive rehabilitation. However, many of those close to Mr. Gage noticed an abrupt change in his personality following the accident. He became brash and aggressive and had difficulties with impulse control, quite unlike the quiet, well-spoken, and thoughtful person they had known before. As his brain healed, his personality continued to change for another 12 years before he died.
The posterior association area controls pattern recognition in a variety of forms. Recognizing someone from their speech patterns as well as visual patterns is put together in the posterior association area. It is also the home to Wernicke’s area, a small region that is considered vital in understanding (and using) spoken and written language. Last, the posterior association area also provides a sense of where we are in space. Combining pattern recognition with information from the somatosensory cortex gives us a sense of where we are in a location and how our body is positioned.
The limbic association area ties emotions to experiences and sensory information. The hippocampus is part of the limbic association area and a key component of it. The hippocampus is a region of gray matter; in humans it can be found in each hemisphere, as one of the walls of the lateral ventricles. This small, nondescript bump of tissue is a key area involved in our understanding of human memory (and one of the first regions to find physical degradation in diseases like Alzheimer’s disease). The hippocampus is responsible for many elements, but there are two that we need to discuss. The first is that the hippocampus helps turn short-term memories into long-term memories, taking sensory information, perception, and emotions and combining them into information that can be recalled at later points to be used in providing context to new information. The second element is that the hippocampus is vital to not just understanding where the body might be in space (that’s the posterior association area) but more importantly how we navigate through that space.
Putting aside the cerebral cortex, the rest of the brain in humans functions quite similarly to that in other mammals and vertebrates. The thalamus connects the cerebrum with the rest of the brain and directs signals to different areas of the cerebrum. The hypothalamus regulates physiological functions, including heart rate, body temperature, water balance, and sleep/wake cycles. This includes physical responses to emotions (blushing when embarrassed, increased heart rate when excited or anxious). The hypothalamus is also a key player in the regulation of the endocrine system function in humans (Chapter 21—Endocrine System). The pons and medulla oblongata generate the rhythms for regular heart rate and respiration. The cerebellum is large in humans and can be visibly separated into two hemispheres along with a vermis. There is a cerebellar cortex and a significant number of gyri and sulci in the cerebellum as well. As in other vertebrates, the cerebellum is responsible for coordinating muscle movements in response to raw signals as well as incoming sensory information.
18.6 Summary
The brain is an extremely complex structure that has changed dramatically across the span of vertebrate evolution. It is the control center of the body, monitoring all the sensory information gathered and directing all voluntary muscle movements. The changes that have occurred are the result of ever-increasing sensory information and changes to how vertebrates move in their environment. Fundamental questions of structure and function have been resolved across many vertebrates, while questions of consciousness, personality, and behavior are still unresolved in our understanding of the brain. New senses, social systems, and even more changes muddy our understanding of the brain, leading to continuing work to this day. The plastic and flexible nature of the brain reminds us that even with smaller groups of vertebrates, there is considerable variation among species.
Application Questions
- Why might the basking shark have a lower relative brain-to-body-mass percentage than the great white or tiger shark? How do their ecology and behavior support your hypothesis?
- How might the brain change in flightless birds like the ostrich or emu?
- Studying the brain (and intelligence and consciousness) has been full of bias and prejudice throughout its history, not just among different human populations, but also when comparing other vertebrates to each other and to humans. How might this have come about as we learned about the brain? How do we know now that many of those “findings” were determined incorrectly?
18.7 Further Reading
- Herculano-Houzel, Suzana. “Birds do have a brain cortex—and think.” Science 369, no. 6511 (2020): 1567–1568.
- Striedter, Georg F., and R. Glenn Northcutt. Brains Through Time: A Natural History of Vertebrates. Oxford University Press, USA, 2020.
18.8 References
- Bloch, Solal, Manon Thomas, Ingrid Colin, Sonya Galant, Elodie Machado, Pierre Affaticati, Arnim Jenett, and Kei Yamamoto. “Mesencephalic origin of the inferior lobe in zebrafish.” BMC Biology 17 (2019): 1–16.
- Budday, Silvia, Paul Steinmann, and Ellen Kuhl. “Physical biology of human brain development.” Frontiers in Cellular Neuroscience 9 (2015): 257.
- Degrange, Federico J., Julieta Carril, Ricardo S. De Mendoza, María M. Demmel Ferreira, and Claudia P. Tambussi. “Anatomy and evolution of avian brain and senses: What endocasts can tell us.” In Paleoneurology of Amniotes: New Directions in the Study of Fossil Endocasts, edited by María Teresa Dozo, Ariana Paulina-Carabajal, Thomas E. Macrini, and Stig Walsh, 333–364. Cham: Springer International Publishing, 2022.
- Eaton, R. C., R. K. K. Lee, and M. B. Foreman. “The Mauthner cell and other identified neurons of the brainstem escape network of fish.” Progress in Neurobiology 63, no. 4 (2001): 467–485.
- Herculano-Houzel, Suzana. “Theropod dinosaurs had primate-like numbers of telencephalic neurons.” Journal of Comparative Neurology 531, no. 9 (2023): 962–974.
- Jerison, Harry. Evolution of the brain and intelligence. Elsevier, 2012.
- Kawakami, Koichi, and Yasunori Murakami. “Preface to vertebrate brains: Evolution, structures and functions.” Development, Growth & Differentiation 59, no. 4 (2017): 160–162.
- Kotrschal, Alexander, and Kurt Kotrschal. “Fish brains: Anatomy, functionality, and evolutionary relationships.” The Welfare of Fish (2020): 129–148.
- Laurent, Gilles, Julien Fournier, Mike Hemberger, Christian Müller, Robert Naumann, Janie M. Ondracek, Lorenz Pammer et al. “Cortical evolution: Introduction to the reptilian cortex.” Micro-, Meso- and Macro–Dynamics of the Brain (2016): 23–33.
- Loonen, Anton JM, and Svetlana A. Ivanova. “Circuits regulating pleasure and happiness: The evolution of the amygdalar-hippocampal-habenular connectivity in vertebrates.” Frontiers in Neuroscience 10 (2016): 539.
- Miterko, Lauren N., Elizabeth P. Lackey, Detlef H. Heck, and Roy V. Sillitoe. “Shaping diversity into the brain’s form and function.” Frontiers in Neural Circuits 12 (2018): 83.
- Mokhtar, Doaa M. “Patterns of organization of cerebellum and spinal cord of the Red-Tail Shark (Epalzeorhynchos bicolor): Histological, morphometrical, and immunohistochemical studies.” Microscopy and Microanalysis 26, no. 6 (2020): 1255–1263.
- Northcutt, R. Glenn. “Understanding vertebrate brain evolution.” Integrative and Comparative Biology 42, no. 4 (2002): 743–756.
- Roth, Gerhard, and Ursula Dicke. “Evolution of the brain and intelligence.” Trends in Cognitive Sciences 9, no. 5 (2005): 250–257.
- Salas, Carlos A., Kara E. Yopak, Rachael E. Warrington, Nathan S. Hart, Ian C. Potter, and Shaun P. Collin. “Ontogenetic shifts in brain scaling reflect behavioral changes in the life cycle of the pouched lamprey Geotria australis.” Frontiers in Neuroscience 9 (2015): 251.
- Streeter, George L. “The structure of the spinal cord of an ostrich.” American Journal of Anatomy 3 (1904): 1–27.
- Sugahara, Fumiaki, Juan Pascual-Anaya, Yasuhiro Oisi, Shigehiro Kuraku, Shin-ichi Aota, Noritaka Adachi, Wataru Takagi et al. “Evidence from cyclostomes for complex regionalization of the ancestral vertebrate brain.” Nature 531, no. 7592 (2016): 97–100.
- Wilczynski, W. “Evolution of the brain in amphibians.” Encyclopedia of Neurosciences (2009): 1301–1305.

