13 Digestive System
Samantha C. Leigh and Donovan P. German
Focus Questions—to Guide Your Reading of This Chapter
- How do the morphology and organization of digestive organs, such as the stomach and intestines, differ among vertebrates, and how does this impact digestive efficiency? Are all herbivores the same?
- How do vertebrates adapt their digestive systems to process diverse diets, ranging from plant matter to animal tissue?
- What are the key structural adaptations in the digestive tracts of vertebrates, and how do they reflect dietary preferences and ecological niches?
- What are the roles of digestive enzymes and secretions in breaking down macromolecules into absorbable nutrients, and how do they vary across vertebrate species?
- What insights can comparative digestive anatomy provide into the evolutionary relationships and ecological interactions among vertebrate taxa?
13.1 Introduction
The digestive system is a fundamental physiological system present in all vertebrates, encompassing a series of specialized organs and tissues tasked with the acquisition, processing, and absorption of nutrients essential for growth, maintenance, and energy production. From the smallest fish to the largest mammals, vertebrates exhibit an astonishing diversity of dietary preferences and feeding strategies, each accompanied by anatomical and physiological adaptations within the digestive tract.
At its core, the vertebrate digestive system follows a general blueprint, comprising a sequence of interconnected compartments and tubes, including the oral cavity, pharynx, esophagus, foregut chamber (if present), stomach (if present), small intestine, large intestine/colon, hindgut caecum (if present), rectum, and anus (Figure 13.1). Associated accessory organs such as the salivary glands, liver, gallbladder, and pancreas contribute additional digestive secretions and enzymes essential for efficient nutrient processing. The complexity and versatility of the vertebrate digestive system reflect the diverse ecological niches occupied by different species as well as the evolutionary pressures imposed by varying dietary regimes. Carnivorous vertebrates, for example, are equipped with adaptations geared toward the intake of sometimes large amounts of protein and the rapid breakdown and assimilation of proteins and fats (Figure 13.1). These adaptations often include relatively short digestive tracts optimized for swift digestion and absorption of nutrients as well as specialized enzymes and gastric secretions tailored to protein-rich diets. Conversely, herbivorous vertebrates face the challenge of extracting nutrients from plant material, which can be structurally complex, low in protein and lipids, and difficult to digest. To overcome this challenge, herbivores have evolved elaborate digestive strategies, some including physical breakdown of plant cell walls and others including prolonged retention times in the digestive tract, extensive microbial fermentation chambers, and symbiotic relationships with microbial communities capable of breaking down plant material (e.g., cellulose) that is otherwise indigestible by the animal’s own digestive enzymes. These adaptations allow herbivores to extract maximal nutritional value from plant material and thrive on diets rich in fiber and carbohydrates (Figure 13.1).
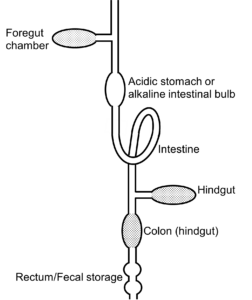
Figure 13.1—Basic design of a vertebrate gut. Most animals have some form of an intestine but vary as to whether they possess other compartments such as crop, foregut chamber, stomach, or caecum. As a general rule, catalytic enzymatic reactions occur in the intestine, whereas microbial fermentation can occur in the foregut, caecum, and large intestine / colon. Foregut fermentation is known to occur in four major clades of mammals and in at least one avian species (the hoatzin). Hindgut fermentation, in either the caecum or large intestine/colon, occurs in many clades of mammals, birds, reptiles, and fishes. Modified from German and Rose (2021).
Furthermore, the vertebrate digestive system is intimately intertwined with other physiological systems, including the circulatory, respiratory, and endocrine systems. The circulatory system, for instance, transports absorbed nutrients from the digestive tract to tissues throughout the body, ensuring a constant supply of energy and building blocks for growth and repair. Hormones secreted by the endocrine system regulate various aspects of digestion, including enzyme secretion, gut motility, and appetite control, orchestrating physiological processes to maintain metabolic homeostasis.
Despite the remarkable diversity of vertebrate species and their dietary preferences, certain fundamental principles of digestive physiology remain conserved across taxa. The sequential processing of food through the digestive tract, the enzymatic breakdown of complex nutrients into absorbable molecules, and the selective absorption of essential nutrients while eliminating waste products are universal features of vertebrate digestion. By studying the comparative anatomy and function of the vertebrate digestive system, researchers gain valuable insights into the evolutionary history of organisms, the ecological interactions shaping biodiversity, and the remarkable adaptability of life in its myriad forms.
13.2 Overview of the Vertebrate Digestive System
The oral cavity serves as the entry point for food and the initial site of mechanical and chemical digestion. Most extant vertebrates have jaws (with hagfishes and lampreys providing the exceptions), and those jaws can have extensive dentition. Teeth are specialized structures composed of enamel, dentin, and cementum that mechanically break down food into smaller, more manageable pieces through the process of mastication. Different types of teeth—such as incisors, canines, premolars, and molars—are adapted for cutting, tearing, and grinding food depending on the diet of the organism (see Section 8.6). In many fishes, the dentition associated with the oral jaws is limited to grasping prey, as most fish do not chew in the oral cavity. Fishes also possess pharyngeal jaws in their pharynx (see Section 8.5) that can have extensive teeth and grinding plates, where food is masticated.
Many vertebrates have a tongue or something similar, which is a muscular organ covered with papillae that assists in the manipulation of food during chewing and helps propel the food bolus (i.e., a semisolid mass of food) toward the pharynx for swallowing. Taste buds located on the surface of the tongue detect various flavors, allowing organisms to assess the palatability of food items. Many fishes have taste buds on their body.
Mammals and some other tetrapod groups have oral glands that secrete mucus (and in the case of mammals, saliva) into the oral cavity. Saliva is composed primarily of water, electrolytes, mucus, and enzymes such as salivary amylase. Salivary amylase begins the chemical breakdown of starches into maltose, a disaccharide composed of two glucose molecules. Because they live in water, the most speciose vertebrate group (fishes) does not produce saliva.
The esophagus is a muscular tube that connects the pharynx to the stomach (if present) and serves as a conduit for the passage of food from the oral cavity to the stomach (Figure 13.1). Peristaltic contractions, coordinated waves of muscular contraction and relaxation, propel the food bolus downward through the esophagus toward the alimentary canal. The esophagus contains two specialized sphincter muscles: the upper esophageal sphincter (UES) at the junction with the pharynx and the lower esophageal sphincter (LES) at the junction with the stomach. The UES prevents air from entering the esophagus during respiration, while the LES prevents the reflux of stomach contents into the esophagus.
Many bird species have a crop, which is a small pouch that precedes the stomach (Figure 13.1). The crop can serve as a space for food storage and releases its contents into the stomach, allowing the animal to ingest a relatively large amount of food and move to another location quickly to engage more in the digestive process. In some species, like pigeons, the crop can secrete milk-like fluids that can be fed to juveniles instead of food acquired from the environment. Moreover, in one bird species called the hoatzin, the crop has been modified into a foregut fermentation chamber, which facilitates the breakdown of hard-to-digest foods like plants.
The stomach is a muscular organ located in the upper abdomen, primarily responsible for the storage, mixing, and initial digestion of food (Figures 13.1 and 13.2). Stomachs have four main tissue layers, from internal to external: mucosa, submucosa, muscularis, and serosa (Figure 13.2). In mammals, the stomach consists of three main regions: the fundus, antrum, and pylorus (Figures 13.2 and 13.3A). Gastric glands within the mucosa of the stomach secrete gastric juice, a mixture of hydrochloric acid (HCl), pepsinogen, mucus, and intrinsic factor (a protein that helps with vitamin B12 absorption in the intestine). Hydrochloric acid creates an acidic environment (pH 1.5–3.5) in the stomach, which activates pepsinogen to its active form, pepsin. Pepsin catalyzes the hydrolysis of peptide bonds in proteins, breaking them down into smaller peptides. The submucosa largely supports the mucosa and connects it to the muscularis. The stomach’s muscular walls (muscularis layer) contract rhythmically, mixing food with gastric juices to form a semifluid mixture called chyme. The stomach also acts as a temporary reservoir for food storage, regulating the rate at which chyme is released into the small intestine for further digestion and absorption. In birds, reptiles, and some fishes, a gizzard-like stomach can also triturate food, reducing particle size by vigorous muscular contractions, causing significant mixing. This process is sometimes aided by the presence of gastroliths, ingested sand or gravel against which stomach material is ground. Nearly 8,000 species of fishes, as well as platypus and echidna among the mammals, have lost the gastric stomach as part of their digestive system. They have an esophagus that empties either straight into the intestine or into an intestinal bulb (e.g., parrotfishes). These species eat a wide array of foods, including carnivorous diets, and function without a stomach. Many species of fish have what are called pyloric caeca immediately following the stomach, which are blind sacs protruding from the intestine and really are an extension of the intestine in terms of function.
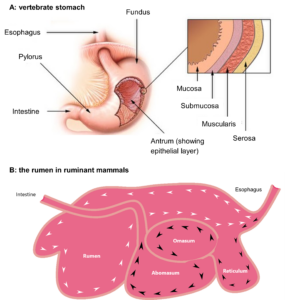
Figure 13.2—A: Illustration of a mammalian (vertebrate) stomach with the inset showing the tissue layers of mucosa, submucosa, muscularis, and serosa. B: Illustration of the rumen in a ruminant mammal (e.g., a cow). Ingesta enters the rumen via the esophagus. When first ingested, most grass particles are buoyant and follow the white arrows, circulating through the rumen, where contact with microorganisms and their enzymes will digest the grass. As particles become smaller and denser (black arrows), they eventually sink into the reticulorumen (reticulum) and are channeled into the omasum, followed by the abomasum (stomach). Microorganisms also escape the rumen into the omasum and abomasum, where they are digested by the host’s enzymes. Microorganisms are the protein sources for ruminants, which by nature consume a low-protein diet.
The small intestine is the longest section of the digestive tract and, like the stomach, has the four tissue layers of mucosa, submucosa, muscularis, and serosa (Figures 13.4 and 13.5). In mammals, the small intestine is divided into three segments noted as the duodenum, jejunum, and ileum. However, these terms have specific anatomical meanings in mammals that do not translate directly to other vertebrates; hence, these terms are not universal. The small intestine (or just intestine) plays a critical role in the absorption of nutrients from digested food. Upon entering the proximal intestine (duodenum in mammals), chyme is mixed with bile from the liver and secretions from the pancreas (including buffer and enzymes) and is now called digesta (see below for more information on the liver and pancreas). The inner surface of the small intestine is highly specialized for nutrient absorption, characterized by numerous finger-like projections called villi (or folds; singular: villus) and microvilli (Figures 13.4 and 13.5). Note that the term villus is a mammalian term, whereas many other vertebrates have intestinal folds and not true villi. Villi increase the surface area available for absorption, while microvilli, composing the highly convoluted membrane of epithelial cells, further enhance nutrient uptake by increasing the absorptive surface area by orders of magnitude (Figures 13.4 and 13.5). Absorbed nutrients—including amino acids, fatty acids, monosaccharides, vitamins, and minerals—are transported across the epithelial cells of the villi into the bloodstream or lymphatic system for distribution throughout the body (Figure 13.4).
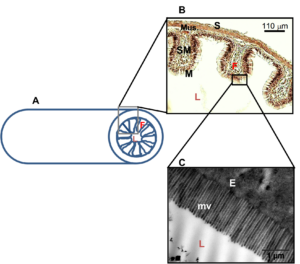
Figure 13.3—General structure of an intestine. Organism: Pterygoplichthys disjunctivus (armored catfish) A: Illustration of a cross section of an intestine showing the intestinal folds along the mucosal lining of the intestine that increase the surface area. B: Light micrograph of a histological preparation of the midintestine of Pt. disjunctivus showing the intestinal folds. Enterocytes are visible lining the fold. C: Transmission electron micrograph of the microvilli on the surface of epithelial cells of the midintestine of Pt. disjunctivus. The microvilli further increase the surface area of the intestine. E = enterocyte; F = intestinal fold; L = lumen of intestine; M = mucosal layer composing the intestinal epithelium; Mus. = muscularis; mv = microvilli; S = serosa; SM = submucosa. Modified from German and Herrera (2024).
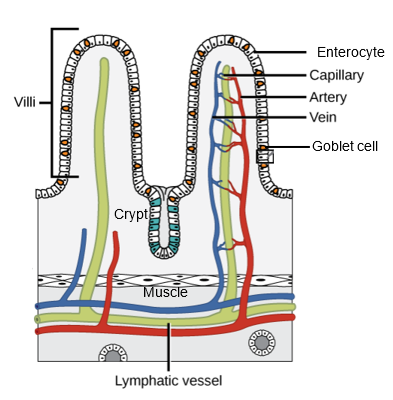
Figure 13.4—General anatomy of an intestine, showing two villi (folds). Note that each villus is covered in enterocytes (the main absorptive cells of the intestine), and goblet cells, which secrete mucus to protect the epithelial cells. These cells are first synthesized in the crypt and migrate up the villus until they reach the tip. Once they reach the tip, they are typically sloughed off by those cells pushing up from below, and thus, enterocytes only have a life of approximately one week. There are also endocrine cells in the crypt (blue). The same tissue layers shown in Figure 13.2 are also visible here, with the enterocyte cells forming the mucosa, the underlying blood and lymph tissue the submucosa, the muscle cells the muscularis, and the basement membrane the serosa.
The large intestine (also called the colon, distal intestine, or hindgut, depending on the animal group) has many functions, including water and electrolyte absorption, formation of feces, and housing of the gut microbiota (Figures 13.1 and 13.2). As digesta passes through the large intestine, the absorption of water and electrolytes forms more solid waste material. The large intestine also provides a habitat for a diverse microbial community known as the gut microbiota, which can reside in the hindgut itself or primarily in the caecum (plural: caeca), when present (Figures 13.1 and 13.2). Note that the human appendix is the remnant of what was likely a larger caecum (as in chimpanzees) before humans started cooking their food, essentially predigesting it before consuming it. Symbiotic microorganisms in the hindgut and/or caecum play essential roles in fermentation of indigestible carbohydrates, synthesis of vitamins (e.g., vitamin K, B vitamins) and amino acids (e.g., lysine), and modulation of immune function. Fecal matter is stored in the rectum until it is eliminated through the anus during defecation, a process controlled by the relaxation of the internal anal sphincter and voluntary contraction of the external anal sphincter.
Chondrichthyans and some nonteleost fishes have a spiral intestine in the latter part of the intestine. This is a specialized segment of the intestine with regions that are analogous to the small intestine and large intestine of other vertebrates. The morphologies of the spiral intestine come in four main forms: an actual spiral that resembles a spiral staircase, a scroll valve that mimics a rolled scroll of paper with epithelial surface on both sides, funnels oriented anteriorly, and funnels oriented posteriorly. This segment of the intestine is absorptive, with elevated surface area, intestinal enzymatic activities, and nutrient transporters. There is also evidence that the flow of material slows in the spiral intestine, thus allowing for efficient digestion and absorption.
The pancreas, liver, gallbladder, and (when present) salivary glands are accessory organs that contribute essential digestive secretions and enzymes to the digestive process. We have already discussed salivary glands, so now we will focus on the other accessory organs. You can also find more information about the pancreas in Chapter 21 and about the specific enzymes in Section 13.4.
The pancreas, located behind the stomach in those animals with a discernible pancreas, secretes pancreatic enzymes—amylase, lipase, and several proteases such as trypsin and chymotrypsin—into the proximal small intestine to aid in the digestion of carbohydrates, lipids, and proteins. The pancreas also secretes bicarbonate ions to neutralize acidic chyme entering the intestine from the stomach, creating a more favorable environment for enzyme activity. Most fish species lack a true pancreas and instead have acinar and islet cells distributed among liver tissue and along the intestine.
The liver, the largest internal organ in vertebrates, contains cells called hepatocytes that secrete bile, a complex fluid containing bile salts, cholesterol, and bilirubin. Bile is stored and concentrated in the gallbladder before being released into the proximal intestine to emulsify lipids for digestion and absorption. Just like they lack other organs, many fish species lack a gallbladder and simply secrete bile straight from the liver into the intestine. Bile salts tend to be reabsorbed in the large intestine (hindgut), where their status as conjugates can act as a feedback to the liver on the physiological state of the animal.
By coordinating the functions of these specialized organs and structures, the vertebrate digestive system efficiently breaks down ingested food into absorbable nutrients while facilitating the elimination of waste products, ensuring the organism’s survival and well-being.
13.3 Structural Adaptations for Digestion
The vertebrate digestive system exhibits remarkable structural adaptations tailored to the dietary preferences and ecological niches of different species. These adaptations include modifications in organ size, shape, and functionality, as well as the presence of specialized structures optimized for specific digestive processes.
Carnivorous vertebrates tend to share many similarities in their general digestive tract structure. For instance, domestic cat (Felis catus), gray wolf (Canis lupus), monitor lizards, and carnivorous fish (e.g., tuna) all have digestive systems optimized for processing animal-based diets rich in protein and fats. Their digestive tracts typically exhibit relatively short lengths compared to herbivores, reflecting the carnivores’ processing of relatively digestible animal tissues. The stomach of carnivorous vertebrates tends to be highly acidic, with a low-pH environment maintained by the secretion of hydrochloric acid (HCl) from gastric glands. This acidic environment aids in the denaturation of proteins and the activation of pepsinogen to pepsin, facilitating the breakdown of proteins into peptides. Carnivorous vertebrates tend to be efficient at metabolically processing low-carbohydrate diets. Many also possess well-developed teeth (e.g., canines) for gripping and tearing flesh, and mammals can also have sharp molars for shearing and crushing bones. Some carnivorous fishes have specialized pharyngeal tooth plates for crushing and grinding animal material, including pulverizing molluscan shells. These specialized dentitions allow carnivores to efficiently process meat and bone tissues, extracting essential nutrients while discarding indigestible materials.
Herbivorous vertebrates, such as horse (Equus caballus), cattle (Bos taurus), the hoatzin (Ophisthocomus hoazin), green iguana (Iguana iguana), and sea chub fish (Kyphosus sp.), have evolved digestive systems specialized for the consumption and digestion of plant-based diets rich in fiber and complex carbohydrates (Figure 13.1). Their digestive tracts often feature elongated intestines and enlarged microbial fermentation chambers (foregut for the cow and hoatzin, hindgut for the others; Figures 13.1–13.2), facilitating the breakdown of otherwise indigestible plant material (including cellulose in some and mannitol in others). Foregut fermenters, including the ruminants like cows and deer and the hoatzin (an avian herbivore), possess a foregut fermentation chamber. In the ruminants, the foregut is divided into a reticulorumen, rumen, omasum, and abomasum (Figure 13.2). The reticulorumen and rumen are the main locations of microbial symbionts that digest plant structural polysaccharides and other complex carbohydrates under anaerobic conditions, producing short chain fatty acids (SCFAs, like acetate, propionate, butyrate), which can be absorbed and utilized as energy sources by the host animal. Only the abomasum is a true “stomach” that produces hydrochloric acid and pepsinogen, digesting the proteins produced by the microbes inhabiting the reticulorumen and rumen. Thus, it is a misnomer to state that ruminants have a “four-chambered stomach”; they only have one stomach (abomasum), and the remainder of the foregut is specialized for fermentation. The hoatzin independently evolved an enlarged crop that is a foregut fermentation chamber where microbes digest the fibrous compounds of plant leaves, producing SCFAs for the host. The crop of the hoatzin is so large that the bird has reduced flight muscles and cannot fly for long distances. Other mammalian groups (some kangaroos, sloths) have foregut fermentation chambers. Hindgut fermenters have large caeca and or distal intestines (colon) where microbial fermentation occurs (Figure 13.1). These fermentation chambers allow hindgut fermenters to extract additional nutrients from plant material that they could not obtain with their own digestive enzymes. There are no known foregut fermenting fishes, so those that do rely on microbial fermentation are hindgut fermenters.
It is noteworthy that not all vertebrate herbivores have symbionts to aid in the digestion of plant material. The giant panda (Ailuropoda melanoleuca) provides a perfect example of an animal that consumes fibrous plant material (bamboo) but does not digest much of the fiber with the aid of microbial symbionts. Instead, giant pandas are considered macronutrient carnivores that target protein from the ingested plant material. The low protein content of the panda diet requires that they eat copious amounts of bamboo to meet their nutritional needs, but they pass this material relatively quickly through an elongated intestine, where protein and soluble carbohydrates are efficiently digested. Many herbivorous fishes, including grass carp, minnows, and armored catfishes, also take this approach of high intake of low-protein food, efficient digestion of protein and soluble carbohydrates, and little reliance on microbes to ferment plant material in their guts.
Omnivorous vertebrates, such as humans (Homo sapiens) and brown bear (Ursus arctos), and numerous bird, reptile, and fish species, consume a diverse diet consisting of both plant and animal matter. Their digestive systems exhibit a combination of adaptations suited for processing a wide range of food types and tend to be more of intermediate length (Figures 13.1 and 13.5). The human digestive system, for example, features an acidic stomach environment optimized for the digestion of both proteins (e.g., meat) and carbohydrates (e.g., plant-based foods). The small intestine, with its extensive surface area provided by villi and microvilli (Figures 13.3 and 13.4), efficiently absorbs nutrients from diverse food sources, including amino acids, fatty acids, sugars, and vitamins. Some omnivores still enlist the aid of microbial symbionts to aid in the digestion of plant material, whereas others do not. However, any of the features that are relatively extreme in carnivores or herbivores are not as pronounced in omnivores (Figure 13.5).
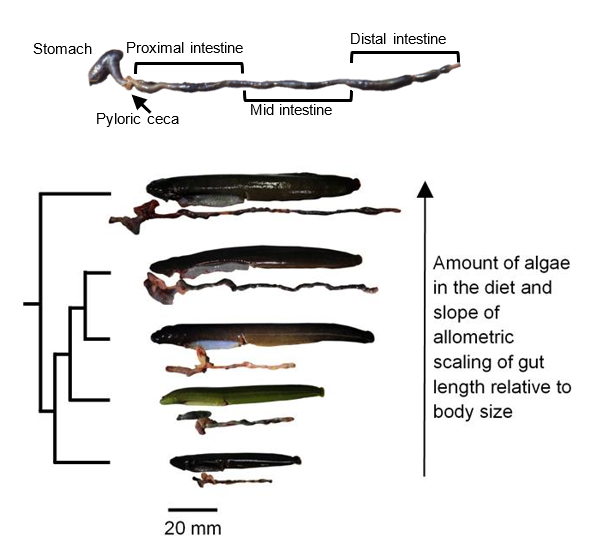
Figure 13.5—Top: The digestive system of the fish Cebidichthys violaceus (family Stichaeidae) showing the stomach, pyloric caeca, and intestine. Bottom: Trimmed phylogeny and photos of fishes (with their unraveled guts beneath them) in the family Stichaeidae, showing a gradient in the amount of algal material in the diet and gut length relative to body size. Increasing the amount of refractory material in the diet leads to higher intake, leading to more rapid transit of digesta through the gut, requiring a longer gut to maintain digestive efficiency (German and Herrera, 2024; Leigh et al., 2021). From top to bottom: Cebidichthys violaceus (herbivore), Xiphister mucosus (herbivore), X. atropurpureus (omnivore), Phytichthys chirus (omnivore), Anoplarchus purpurescens (carnivore).
Some vertebrates have evolved specialized feeding habits and digestive adaptations to exploit unique ecological niches. For example, frugivores, such as fruit bats and some primates, have adaptations for consuming fruits, including specialized dentition for biting and crushing fruits as well as an elongated digestive tract optimized for processing plant material and seeds. Some frugivorous and nectivorous (nectar-drinking) bats and birds achieve blood glucose levels that would pose physiological challenges in other vertebrates, yet they can tolerate these high solute concentrations in their blood. Insectivores, such as anteaters and some birds, have adaptations for capturing and consuming insects, including long, sticky tongues for capturing prey and a relatively short digestive tract optimized for processing high-protein insect diets. Piscivores, such as dolphins, penguins, and some fish species, have adaptations for capturing and consuming fish, including sharp teeth and streamlined bodies for efficient swimming as well as expandable stomachs capable of rapid digestion of fish proteins and fats. Carnivorous marine mammals have relatively long intestines relative to their body sizes in comparison to terrestrial mammalian carnivores, and this is perhaps to aid in water absorption, since these animals do not drink seawater and must obtain their water from ingested food.
Overall, the structural adaptations observed in the vertebrate digestive system reflect the diverse dietary preferences and ecological strategies employed by different species. These adaptations highlight the remarkable versatility and adaptability of vertebrates in exploiting various food resources and habitats throughout the natural world.
Box 13.1—Make a Model of a Digestive System
Are all guts the same? Take a moment to do online searches of three different vertebrates (preferably not from the same phylogenetic group—for instance, don’t pick a cow and a deer) and find images of their digestive tracts. What segments of the system do they have? Does each have a stomach? What about a caecum in their hindgut? Why is there variation among their guts? If you have time, try drawing or building a model digestive system. What did you include in your model and why? What do you think your model gut would digest well?
13.4 Digestive Enzymes and Secretions
Digestive enzymes serve as catalysts in the digestive process, facilitating the breakdown of complex dietary components into simpler forms that can be absorbed and utilized by the body. Each enzyme is specialized to target specific substrates, initiating biochemical reactions that result in the hydrolysis of bonds within macromolecules. An overview of enzymes can be found in Figure 13.6.

Figure 13.6—Principal nutrients in food and the endogenous digestive enzymes vertebrates produce to hydrolyze them. Digestion requires multiple steps, beginning with hydrolysis in the lumen by extracellular enzymes and followed by membrane digestion by intestinal mucosal enzymes. For protein, the enzyme pepsin is listed in parentheses because agastric species (i.e., those that lack an acidic stomach) lack this enzyme.
Amylase (pancreatic, and salivary in the case of mammals) initiates the digestion of carbohydrates. This enzyme primarily targets starches and glycogen, which are complex polysaccharides composed of glucose units linked by alpha-1,4 glycosidic bonds (covalent bonds linking sugar molecules to other molecules). Amylase hydrolyzes these bonds, breaking down the polysaccharides into smaller oligosaccharides, maltose, and dextrins. The hydrolysis reaction involves the addition of water molecules to the glycosidic bonds, resulting in the cleavage of the bonds and the release of maltose molecules. Maltose itself is then hydrolyzed by maltase (𝝰-glucosidase) into individual glucose molecules that are absorbed by sodium-dependent glucose transporters (SGLT) embedded in the microvilli of epithelial cells. In some vertebrates, chitin can also be digested by an acidic chitinase followed by N-acetyl-𝜷-D-glucosaminidase, producing monomers of N-acetyl-glucosamine (Figure 13.6). Vertebrates lack the requisite enzymes to digest cellulose and heteropolymers like hemicellulose and thus require the aid of microbial symbionts to digest those compounds (if that is part of the digestive strategy of the animal, again pointing to the panda as an example of an animal that doesn’t rely on symbionts to digest plant fiber).
Pepsinogen, produced by oxynticopeptic cells (chief cells in mammals) in the gastric glands of the stomach, is the inactive precursor of pepsin, a proteolytic enzyme involved in protein digestion. Pepsinogen is secreted into the stomach lumen, where it undergoes proteolytic cleavage by hydrochloric acid (HCl) to form pepsin. The acidic environment of the stomach (pH 1.5–3.5) activates pepsinogen by inducing a conformational change that exposes the active site of the enzyme. Once activated, pepsin catalyzes the hydrolysis of peptide bonds within proteins, specifically targeting peptide bonds adjacent to aromatic amino acids such as phenylalanine and tyrosine. Pepsin cleaves proteins into smaller peptides and amino acids through an endopeptidase mechanism, breaking bonds within the interior of protein molecules. This initial protein digestion in the stomach prepares dietary proteins for further enzymatic hydrolysis in the small intestine, facilitating the absorption of amino acids and peptides. Given that the gastric stomach evolved approximately 450 million years ago, this combination of acid hydrolysis, combined with an endopeptidase like pepsin, has been an effective tool with which vertebrates digest protein in the stomach.
As briefly mentioned above, the pancreas secretes a variety of digestive enzymes into the proximal intestine (duodenum in mammals), the first segment of the small intestine, in response to hormonal signals triggered by the presence of food. These enzymes play crucial roles in the digestion of carbohydrates, proteins, and lipids.
Amylase: Pancreatic amylase digests carbohydrates. It hydrolyzes the alpha-1,4 glycosidic bonds present in starches and glycogen, breaking them down into maltose, maltotriose, and oligosaccharides. Amylase exhibits optimal activity at a slightly alkaline pH, allowing for efficient carbohydrate digestion. Amylase activities have been shown to be higher in those animals consuming more starches such as in mammals (including humans), birds, and fishes, and many of these abilities are linked to an increased number of copies of the amylase gene in the genomes of those animals. Note that pancreatic amylase (amy2), which is in all known vertebrate animals, is a different gene and protein from salivary amylase (amy1) in mammals.
Lipase: Lipase (pancreatic in mammals, and carboxyl ester lipase in other vertebrates) is essential for the digestion of dietary fats (triacylglycerides). Lipase catalyzes the hydrolysis of ester bonds present in the glycerol backbone of triacylglyceride molecules, releasing free fatty acids and monoacylglycerides. This process results in the formation of micelles, small aggregates of lipids, bile salts, and other components, which enhance the absorption of lipids by the intestinal epithelium.
Proteases: Pancreatic proteases, including trypsin, chymotrypsin, and carboxypeptidase, are responsible for protein digestion. Trypsin hydrolyzes peptide bonds adjacent to positively charged amino acids such as lysine and arginine, while chymotrypsin targets bonds adjacent to aromatic amino acids like phenylalanine and tyrosine. Carboxypeptidase cleaves peptide bonds at the carboxyl end of peptides and proteins. These proteases collectively break down dietary proteins into smaller peptides and amino acids, facilitating their absorption in the small intestine.
Pancreatic duct cells and proximal intestinal cells produce sodium bicarbonate buffer to raise the pH of acid chyme as it enters the proximal intestine from the stomach. This is not necessary in agastric vertebrates like carps, wrasses, echidna, and platypus.
With the exception of lipases, which already release the appropriate monomeric units of fatty acids and glycerol, the other polymer-degrading digestive enzymes (e.g., amylase, trypsin) produce oligomers and dimers that need to be hydrolyzed further to generate the monomers that are absorbed across the epithelial membrane. Disaccharidases like maltase and N-acetyl-𝜷-D-glucosaminidase, as well as aminopeptidases, are embedded in the mucosal membrane in the microvilli (Figure 13.3) and perform the final hydrolytic step before monomer absorption. Thus, polymer degradation is a complex process that involves an enzymatic cascade to deconstruct complex molecules down to absorbable monomers. Vertebrates invest approximately 25% of their daily energy budgets to digestive tract function, and thus, this is an important aspect of their daily lives.
Bile, produced by hepatocytes in the liver and stored and concentrated in the gallbladder, aids in lipid digestion and absorption. Bile contains bile salts (derivatives of cholic acid), which act as emulsifiers to break down large lipid droplets into smaller droplets known as micelles. This process, called emulsification, increases the surface area of lipid particles, making them more accessible to pancreatic lipase for enzymatic hydrolysis. Bile salts have hydrophobic and hydrophilic regions, allowing them to associate with both water and lipids, thereby stabilizing the emulsified lipid droplets. The formation of micelles facilitates the hydrolysis of triglycerides by pancreatic lipase, leading to the release of fatty acids and monoglycerides that can be absorbed by the intestinal epithelium.
13.5 Digestive System Evolution and Diversity
All animals have the inner tube of life running down the center of their bodies. The need to acquire nutrients, energy, vitamins, and minerals from ingested foods is obvious in heterotrophic organisms. Digestive tract size and diversity of compartments are apparent as one begins to examine guts in different animals. From the ruminant mammals, to stomachless fishes, to flying vertebrates, gut morphology shows wide variation. So too does function, with more or less reliance on microbial symbionts, and wide variation in digestive enzyme activities and transport rates, depending on diet. In this section, we will focus on some of the extreme examples—namely, the smaller guts of flying vertebrates (including birds and bats) and the enigmatic loss of the gastric stomach in over 8,000 species of fishes as well as in the platypus and echidna—and we will finish with the genetics underlying digestive enzyme activity variation in relation to diet. All told, the evolution of trophic and locomotor variation has led to a large diversity in the themes of different gut types (Figures 13.1 and 13.2), each with different consequences for the animals. The diversity of guts is a testament to the fact that there are multiple solutions to solve a single problem.
Flying Vertebrates Have Smaller Digestive Tracts
Imagine you are a sparrow, you’ve just encountered a large number of seeds on the ground, and you quickly gather as many as you can. You see a shadow above you, and you know that it is a potential predator that is now in pursuit. If you don’t quickly leave the area and evade the predator, your life will be cut short. You take off and rapidly fly toward tree cover. Flight, as a mode of locomotion, is expensive; in order to fly, the animal has to be lightweight. Yet the sparrow consumes seeds that are rich in structural polysaccharides and lipids and likely require a somewhat larger gut with which to digest the seeds, as seen in small nonvolant (nonflying) mammals with granivorous diets. So how does a sparrow, or any volant (flying) bird or mammal, achieve a smaller gut so that they are not weighed down yet still efficiently digest their food? Indeed, the adaptation of the gut of volant vertebrates is an interesting case study in how a digestive system can evolve to fit the necessary function.
First of all, flying vertebrates, as a rule, have smaller digestive systems with lower surface areas than nonvolant vertebrates (Figure 13.7), and this difference is more pronounced the smaller the animal gets. By having smaller guts, their alimentary canals don’t weigh as much and thus allow them to be lighter than a comparably sized nonvolant vertebrate. Second, birds have a crop, which is a chamber that precedes the stomach. This allows birds to ingest food items and temporarily store them in the crop before digestion begins. This serves as a way to allow for ingestion and then quick movement to another location before digestion begins, which would serve something small, like a sparrow, well, since it can acquire resources and then retreat to safety before digesting it. Moreover, because many birds regurgitate food items for their young, the crop provides a place to put ingesta (ingested food items) without beginning digestion, thus allowing for nondigested food to be delivered to the young.
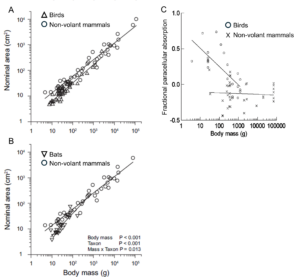
Figure 13.7—(A) Nominal surface area of small intestines of omnivorous birds and nonvolant mammals. Lines were fit to a common slope (0.73), with variable intercepts at line of unity: 1.14 for birds and 1.79 for mammals, showing that birds had 36% lower intestinal surface area than nonvolant mammals. (B) Nominal surface area of bat species compared with the nonflying mammals. (C) Fractional absorption of neutral inert water-soluble compounds for birds and nonvolant mammals.
Third, volant vertebrates, with their shorter guts and lower surface area, make up for this reduction by having greater rates of paracellular absorption. Most vertebrates require transepithelial absorption of most nutrients: Transporters on the brush border membrane of epithelial cells use sodium-dependent transport to bring the nutrients (e.g., glucose, amino acids) into epithelial cells before the nutrients are then transported across the basolateral membrane into the blood for distribution in the body (Figure 13.4). Efficient absorption using this transepithelial route requires high surface area (microvilli) and many transporters in the membrane, both of which are expensive to maintain and lead to more tissue mass. Volant vertebrates have solved that requirement by just allowing more nonspecific absorption between their epithelial cells, as opposed to requiring nutrients to go the transepithelial route. Paracellular absorption is even more important for smaller birds than larger ones (Figure 13.7C), which again points to this strategy being more about having less mass so as to not impede the ability to fly. Of course, nothing is free, and it should be apparent that less control over what is absorbed paracellularly (i.e., it doesn’t have to go through a transporter) comes with a potential cost of absorbing toxins that can harm the animal. Hence this would mean that smaller birds and bats would need to actively avoid toxins or consume compounds that might inhibit the toxicity of various compounds to compensate for this vulnerability, and there is evidence that this is the case.
No Stomach? No Problem!
The gastric stomach is an important vertebrate innovation (Figures 13.2, 13.3, and 13.6). An acidic foregut pouch with sphincters separating it from the esophagus and intestine evolved approximately 450 million years ago, along with oral jaws. The acidic environment along with an acidophilic protease (pepsin) likely improved protein digestion. Moreover, this pouch provided an area for some food storage, so the rate of material entering the intestine could be controlled. Add to that the fact that the acidic environment is an important “gate” determining what microbes reach the intestine, and the stomach may be one of the most important aspects of early vertebrate evolution. The fact that greater than 95% of terrestrial vertebrate species and about 75% of all fishes still have a gastric stomach points to its importance. Alternatively, the fact that this number is not 100% is intriguing. At least 10 times in different groups of fishes, as well as in the platypus and echidna, the stomach has been lost, along with the genes (and proteins) for important transporters, pepsin, and proteins that are key for tight junction formation (claudins). Entire orders of fishes (e.g., Cypriniformes, Atherinoformes), as well as the family Labridae (which includes parrotfishes), encompassing thousands of species of fishes, lack a gastric stomach. These fish clades show incredible dietary diversity, ranging from detritivory and herbivory, to carnivory (including piscivory). Thus, one might assume that it would be more efficient to digest a proteinaceous diet with an acidic gastric compartment, yet these fishes get by just fine. The reasons for stomach loss are not clear, as there is not one consistent argument for why stomach loss would be beneficial. Nonetheless, in each of these events of the stomach disappearing, the same suites of genes disappear from the genome, meaning that the stomach phenotype is very unlikely to “reevolve” in those lineages.
Beyond the fishes, the platypus and echidna also each independently lost their stomachs. Each of these lineages also consumes a lot of animal protein and chitin (insects, insect larvae, worms), the digestion of which would potentially benefit from the stomach. The main thing we can conclude from the loss of the stomach phenotype is that it is not a requirement for the vertebrate digestive process; humans who have had gastric bypass surgery also bear this out. Many stomachless fishes have well-developed pharyngeal teeth to reduce particle size before ingesta reaches the intestine, but this is also not a universal rule. Thus, as we see with various structures of the gut—crop, foregut chamber, stomach, caeca (including the appendix in humans)—the intestine, liver, and pancreas (or at least equivalent pancreatic cell types, if not an entire organ) form the basic requirements of a functional digestive system. Beyond those, different animal groups have solved the issues of digestion of different food types in different ways. One may assume that the proteolytic machinery outside of acid and pepsin, so the pancreatic proteases (trypsin, chymotrypsin, carboxypeptidases), as well as the intestinal aminopeptidases, would be expanded in stomachless vertebrates, and this is a question that has yet to be thoroughly answered and would benefit from a physiological genomics approach comparing relatively closely related species on either side of the loss of the stomach in a given lineage.
The Genetics Underlying Digestive Enzyme Activities
Digestion is a chemical process. Yes, there are physical aspects relating to mastication or trituration, as well as fluid dynamics in the tubular intestinal environment, but by and large, the dismantling of polymers to absorbable monomers is driven by chemistry, and particularly the activity levels of digestive enzymes. When one starts comparing the digestive enzyme activity levels present in different animals with different diets, the first one to jump out as being variable is that of amylase. Amylase activities are elevated in those animals consuming more starch (i.e., herbivores and omnivores) in comparison to those that do not (e.g., carnivores). This pattern has been observed in several mammal groups, including humans, canids (dogs and wolves), mustelids, and bats. Moreover, it is observed in birds and in fishes. The human and canid examples drew much of the attention following the turn of the millennium and the explosion of high-throughput genetic sequencing techniques. It was revealed that humans from agrarian (agricultural) backgrounds had many more copies (up to 14) of the salivary amylase gene (amy1) than hunter-gatherer humans, who had 6. That expanded gene copy number led to more gene expression and thus more amylase protein synthesis and elevated amylolytic activities in those people whose lineages have been eating starchy grains for over 10,000 years. Because we have been feeding dogs grains for almost the same length of time, it shouldn’t be surprising that similar effects were seen in dogs, where they have up to 30 copies of the amy2 gene in comparison to only 2 in wolves.
The elevated amylase in starch-consuming vertebrates doesn’t stop there. Bats consuming more starchy fruits have elevated amylase activities in their guts in comparison to carnivorous bats; birds that consume more grains have greater amylase activities than those that don’t. In fishes, it gets a little more interesting. Herbivorous and omnivorous fishes have elevated amylase activities in comparison to carnivores in many different clades, but what are the genetic underpinnings of this? Is it the same as seen in mammals with massive increases in gene copy number? The answer is sometimes yes and sometimes no. Comparative genomics and transcriptomics of amylase genes in prickleback fishes make it clear that those that consume more algae have elevated amylolytic activities, elevated expression of the genes, and variation in amy2 gene copy number. Those prickleback fishes consuming less algae have one amy copy (two diploid copies), whereas those with higher activities have two to three (depending on species; Figure 13.8). In the two Xiphister pricklebacks, one omnivore and one herbivore, the two copies are identical. However, in Cebidichthys violaceus (the monkeyface prickleback, which is also a herbivore), two copies are identical, but the third copy is different from the other two, and the enzyme has a different isoelectric point and pH optimum, suggesting it operates farther down the intestine with more alkaline conditions. Thus, not only can we see gene copy number variation among species with different diets, but we can also see mutations among the proteins that can cause differences.
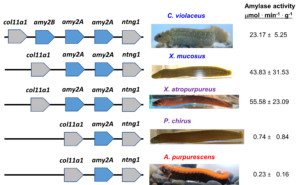
Figure 13.8—Synteny maps showing the copies of amylase (amy2) genes, their orientation, and distribution on the chromosomes of five prickleback species. C. violaceus and X. mucosus are herbivorous, X. atropurpureus is omnivorous, as is P. chirus, but they consume less algae than X. atropurpureus. A. purpurescens is carnivorous. Total gut amylase activities are to the right of the fish pictures (mean ± standard deviation).
Beyond these observations in amylase, other digestive enzymes are not as well scrutinized, but trypsin may show more copy numbers in some carnivores. This leads to questions about lipase. Shouldn’t those also show greater copies in carnivores, which consume more of a lipid-rich diet? The short answer is, it depends on what lipase one is discussing. Mammals have pancreatic lipase, among other lipases, whereas fishes have carboxyl ester lipase as their main lipolytic enzyme. In the prickleback fishes, C. violaceus shows the highest lipase activities and has one more copy of the carboxyl ester lipase gene than the prickleback Anoplarchus purpurescens. Algae are low in lipids, which suggests that C. violaceus has elevated lipase activity to scavenge for scant, and essential, lipids in its algal diet. Much more work is to be done to understand the genetic underpinnings of digestive biochemistry.
Box 13.2—Does Amylase Digest Starch?
Mammals have amylase in their saliva, and you can see it in action with a little chemistry. Did you know that iodine binds to starch, turning a dark-blue color? However, iodine doesn’t bind to smaller sugars (like disaccharides or monosaccharides). Wear gloves for this activity. Your instructor can provide you with a 1% starch solution in a buffer. Add a dropperful of starch to a test tube. Then add five drops of iodine, record the color of the solution, and incubate it for 10 minutes. You should see it turn a dark-blue color over time. Now what happens if you add amylase to that starch solution? For safety reasons, your instructor will provide you with an amylase solution, but your own saliva can do the trick! Add a dropperful of starch to a test tube, then add about half a dropper of amylase solution. Shake it to mix it, and hold the test tube in your hand to provide warmth. Let the amylase and starch interact for about 10 minutes. Then just like before, add five drops of iodine to the starch/amylase mixture. Record the color and wait 10 minutes. After those 10 minutes, which was darker? The solution with the amylase and the starch or just the starch? Why is there a difference? Have a class discussion about what is happening. Under controlled conditions, your instructor can show you what their saliva does to starch (Hint: It will be like the amylase solution). We just cannot have everyone spitting in vials for sanitary purposes! Measuring the actions of digestive enzymes can be fun with a little chemistry.
13.6 Summary
The vertebrate digestive system is a marvel of evolutionary adaptation, reflecting the diverse dietary preferences and ecological niches occupied by different species. Despite variations in structure and function, fundamental principles of digestion, nutrient absorption, and waste elimination are shared across vertebrate taxa. Understanding the comparative anatomy and function of the vertebrate digestive system provides insights into the evolutionary relationships between organisms and the intricate interplay between form and function in biological systems.
Application Questions
- You’ve discovered a fossilized complete theropod dinosaur skeleton. As you examine your discovery, you make note of the fairly wimpy teeth of the specimen, as well as a pile of smooth stones in the ribcage. Given your knowledge of vertebrate evolutionary relationships and the digestive system, what does this suggest about the diet and digestive structures of this species?
- Describe foregut versus hindgut fermentation. What similarities and differences are present in the digestive systems of animals that do each type of fermentation? What about behavioral differences?
13.7 References
- Axelsson, Erik, Abhirami Ratnakumar, Maja-Louise Arendt, Khurram Maqbool, Matthew T. Webster, Michele Perloski, Olof Liberg, Jon M. Arnemo, Åke Hedhammar, and Kerstin Lindblad-Toh. “The genomic signature of dog domestication reveals adaptation to a starch-rich diet.” Nature 495 (2013): 360–364.
- Caviedes-Vidal, Enrique, Todd J. McWhorter, Shana R. Lavin, +2, and William H. Karasov. “The digestive adaptation of flying vertebrates: High intestinal paracellular absorption compensates for smaller guts.” Proceedings of the National Academy of Sciences U.S.A. 104 (2007): 19132–19137.
- German D. P., A. K. Gawlicka, and M. H. Horn. “Evolution of ontogenetic dietary shifts and associated gut features in prickleback fishes (Teleostei: Stichaeidae).” Comparative Biochemistry and Physiology Part B 168 (2014): 12–18.
- German, D. P., and M. J. Herrera. “Digestive efficiency.” In Encyclopedia of Fish Physiology, 2nd ed., edited by Sarah L. Alderman and Todd E. Gillis, 396–407. Oxford: Academic Press, 2024.
- German, Donovan P., and Michael H. Horn “Gut length and mass in herbivorous and carnivorous prickleback fishes (Teleostei: Stichaeidae): Ontogenetic, dietary, and phylogenetic effects.” Marine Biology 148 (2006): 1123–1134.
- German, Donovan P., and Michael R. Rose. “Notes toward an evolutionary biology of nutrition.” In Nutrition, Food, and Diet in Ageing and Longevity, vol. 14, edited by Suresh I. S. Rattan and Gurcharan Kaur, 123–151. Berlin: Springer Nature, 2021.
- German, Donovan P., Aaron Sung, Parth Jhaveri, and Ritika Agnihotri. “More than one way to be an herbivore: Convergent evolution of herbivory using different digestive strategies in prickleback fishes (family Stichaeidae).” Zoology 118 (2015): 161–170.
- Heras, Joseph, Mahul Chakraborty, J. J. Emerson, and Donovan P. German. “Genomic and biochemical evidence of dietary adaptation in a marine herbivorous fish.” Proceedings of the Royal Society B: Biological Sciences 287 (2020): 20192327.
- Herrera, M. J., and D. P. German. “Intestinal microbiome function.” In Encyclopedia of Fish Physiology, 2nd ed., edited by Sarah L. Alderman and Todd E. Gillis, 419–430. Oxford: Academic Press, 2024.
- Karasov, William H., and Angela E. Douglas. “Comparative digestive physiology.” Comprehensive Physiology 3 (2013): 741–783.
- Karasov William H, and Carlos Martínez del Rio. Physiological Ecology: How Animals Process Energy, Nutrients, and Toxins. Princeton: Princeton University Press, 2007.
- Kato, Akira, Supriya Pipil, Chihiro Ota, Makoto Kusakabe, et al. “Convergent gene losses and pseudogenizations in multiple lineages of stomachless fishes.” Communications Biology 7 (2024): 408.
- Lavin, Shana R, and William H. Karasov. “Allometry of paracellular absorption in birds.” Physiological and Biochemical Zoology 81 (2008): 551–560.
- Le, Ninh, Joseph Heras, Michelle J. Herrera, Donovan P. German, and Lisa T. Crummett. “The genome of Anoplarchus purpurescens (Stichaeidae) reflects its carnivorous diet.” Molecular Genetics and Genomics 298 (2023): 1419–1434.
- Leigh, Samantha C., Yannis P. Papastamatiou, and Donovan P. German. “Gut microbial diversity and digestive function of an omnivorous shark.” Marine Biology 168 (2021): 55.
- Leigh, Samantha C., Adam P. Summers, Sara L. Hoffmann, and Donovan P. German. “Shark spiral intestines may operate as Tesla valves.” Proceedings of the Royal Society B: Biological Sciences 288 (2021): 20211359.
- Nie, Yongang G., Fuwen Wei, Wenliang Zhou, Yibo Hu, Alistair M. Senior, Qi Wu, Li Yan, and David Raubenheimer. “Giant pandas are macronutritional carnivores.” Current Biology 29 (2019): 1677–1682.e1672.
- Perry, George H., Nathaniel J. Dominy, Katrina G. Claw, Arthur S. Lee, et al. “Diet and the evolution of human amylase gene copy number variation.” Nature Genetics 39 (2007): 1256–1260.
- Rankins, Daniel R., Michelle J. Herrera, Michelle P. Christensen, Alisa Chen, Newton Z. Hood, Joseph Heras, and Donovan P. German. “When digestive physiology doesn’t match ‘diet’: Lumpenus sagitta (Stichaeidae) is an ‘omnivore’ with a carnivorous gut.” Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology 285 (2023): 111508.
- Stevens, C. Edward, and Ian D. Hume. Comparative Physiology of the Vertebrate Digestive System, 2nd ed. Melbourne: Press Syndicate of the University of Cambridge, 1995.
- Wilson, J. M., and L. F. C. Castro. “Morphological diversity of the gastrointestinal tract in fishes.” In The Multifunctional Gut of Fish, Vol. 30, edited by Martin Grosell, Anthony Farrell, and Colin Brauner, 1–55. San Diego: Elsevier, 2011.
Media Attributions
- Figure 13.1 © Donovan P. German is licensed under a CC BY (Attribution) license
- Figure 13.2 © NIH / National Cancer Institute is licensed under a Public Domain license
- Figure 3 © Donovan P. German is licensed under a CC BY (Attribution) license
- Figure 13.6 © Donovan P. German is licensed under a CC BY (Attribution) license
- Figure 13.7 © Caviedes-Vidal, E, et. al (Non-commercial use permitted per https://doi.org/10.1073/pnas.0405514101)
- Figure 13.8 © Donovan P. German is licensed under a CC BY (Attribution) license

