10 Appendicular Skeleton
Vanessa K Hilliard
Focus Questions—to Guide Your Reading of This Chapter
- Where do the paired appendages come from, starting with fins?
- What are the component structures of fins?
- Where the heck did limbs come from?
- How have limbs diversified across vertebrate taxa?
10.1 Introduction
At this point, you’ve been introduced to the skeletal tissues (Chapter 7), the skull (Chapter 8), and the axial skeleton (Chapter 9). In this chapter, we’ll wrap up the skeletal system by taking a look at the appendicular skeleton, which includes fins and limbs as well as their supporting structures (“girdles”; Figure 10.1). The paired appendages and their girdles are the structures that allow us (and other finned/limbed vertebrates) to propel ourselves and to maneuver in our environments.
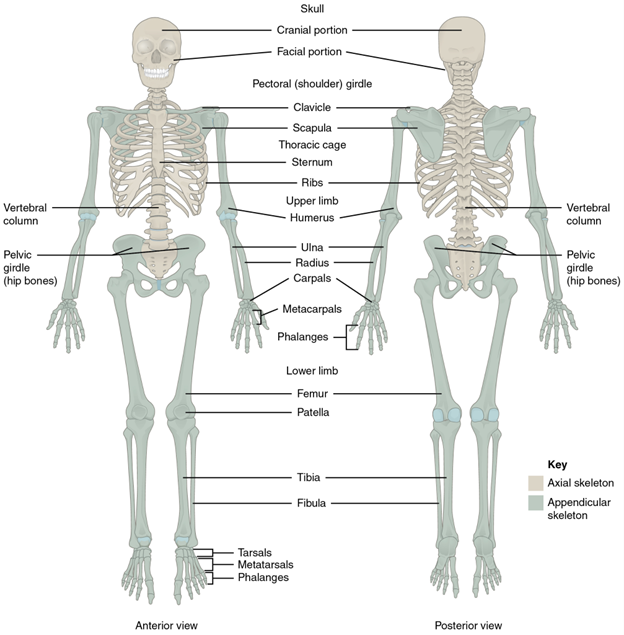
Figure 10.1—The axial and appendicular skeletons of a human. The limbs and girdles are the structures shown in gray.
We’ll begin with a look at where the appendages came from. Then we’ll introduce the component structures of the fins, how we get from fins to limbs, and finally limb diversity in vertebrate animals.
10.2 Development
As you know from the vertebrate diversity chapter (Chapter 3), hagfishes and lampreys have no paired appendages. However, later on the evolutionary timeline, we see the emergence of paired appendages in the gnathostomes. But where did these structures come from, and why did they evolve in the first place? Well, if we observe living fishes that lack paired fins, we find that they are pretty lousy swimmers, and by extension, it is likely that those early fishes prior to the evolution of paired fins would have also been pretty unsteady. So fins initially functioned as a stabilizing mechanism during swimming, and this eventually led to the evolution of paired fins and their associated girdles.
Gnathostomes have two pairs of paired fins, pectoral and pelvic, and both are derived from a chain of basal elements. However, there are two long-standing, competing hypotheses that describe the evolution of these fins. These hypotheses are the gill-arch hypothesis (distinct from the gill-arch hypothesis introduced in the jaws section of Chapter 8), which posits that the fin elements are derived from gill-arch structures, and the fin-fold hypothesis, which posits that the fins are derived from an interruption of two continuous fin folds that ran the length of the body (Figure 10.2).

Figure 10.2—The fin-fold hypothesis (left) suggests the pectoral and pelvic fins evolved from the interruption of paired fin folds that ran the length of the vertebrate body. The gill-arch hypothesis (right) suggests that fin structures are derived from the gill-arch skeleton.
Of these two hypotheses, the fin-fold hypothesis has traditionally had broader support. However, recent work now suggests that both may in fact describe some aspect of fin evolution. Given the conflicting data on the origin of fins from developmental, paleontological, and comparative sources, it is possible that the pectoral appendage in particular may indeed have a dual origin, with the evolution of the pectoral girdle explained by the gill-arch hypothesis and the pectoral appendage explained through the fin-fold hypothesis. Given the far posterior position of the pelvic fin and girdle, there is little dispute that the pelvic structures are likely explained through the fin-fold hypothesis.
10.3 General Structure and Function
If we were to look at the structure of the fin itself, we’d see that distally, the fins are supported by skeletal rays. In chondrichthyans, these rays are made of cartilage and called ceratotrichia. However, in osteichthyans, the distal fin rays are made of dermal bone and called lepidotrichia. The fin rays—both ceratotrichia and lepidotrichia—are supported proximally by endochondral condensations called pterygiophores. Typically, there’s a distal group and a proximal group of pterygiophores. The distal elements, close to the lepidotrichia, are called the radial pterygiophores, whereas the proximal elements that support the radials are called the basal pterygiophores (Figure 10.3).
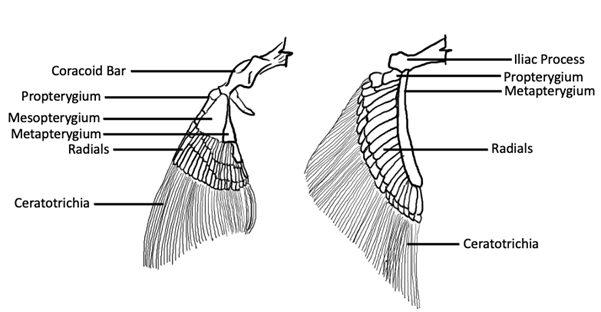
Figure 10.3—Chondrichthyan pectoral (left) and pelvic fins (right), showing positional relationships of ceratotrichia, radials, and basals. Adapted from Liem et al., 2001.
Ancestrally, in both chondrichthyans and actinopterygians, there were three basal elements: propterygium, mesopterygium, and metapterygium (Figure 10.3). As a result, the fins of these lineages are called tribasal (think: tri means “three,” so “three basals”), and it is thought that as fins became larger through the course of evolution, pterygiophores from each side extended proximally and medially and ultimately coalesced to contribute to the formation of the girdles.
The pelvic girdle is made entirely of endochondral bone, and ancestrally it does not attach to the vertebral column (Figure 10.4). The pelvic girdle is typically a single element, but it can have three distinct portions or spokes:
- Dorsally: iliac portion
- Anteroventrally: pubic portion
- Posterioventrally: ischiac portion
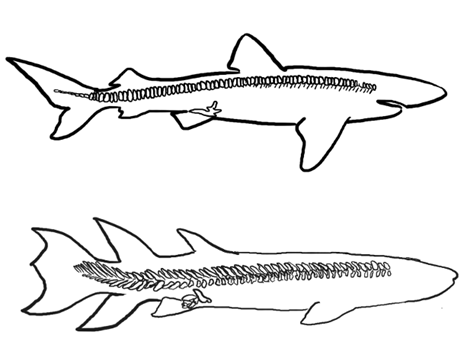
Figure 10.4—Pelvic girdle in chondrichthyan (top) and osteichthyan fishes (bottom). Notice the position of the pelvic girdle relative to the vertebral column (detached) in both taxa.
The pectoral girdle also incorporates several overlying dermal bones from the posterior cranial region (except in chondrichthyans, due to the loss of dermal bone in this group). These bones function to attach the pectoral appendages to the back of the head, meaning that, functionally, the shoulders and the head are anchored to one another. The endochondral portion of the pectoral girdle is sometimes a single element called the scapulocoracoid, but it may also be formed from discrete scapular and coracoid condensations in some taxa. The dermal portion of the pectoral girdle has several components (Figure 10.5):
- The aforementioned scapulocoracoid articulates with the largest of these, called the cleithrum.
- Ventrally, there’s a small clavicle, and in the sarcopterygians, there is sometimes a midline interclavicle; these structures unite the girdles from left and right sides.
- Dorsally, there are supracleithra and postcleithra; these articulate with the posttemporal bone that connects to the skull.
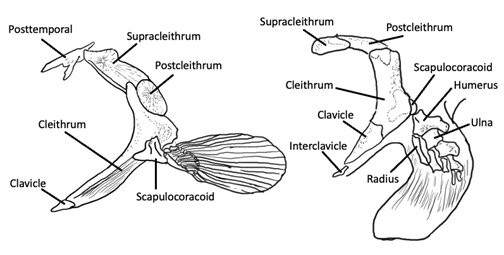
Figure 10.5—Pectoral girdle of basal osteichthyan (Amia, left) and sarcopterygian (right). Adapted from Liem et al., 2001.
Another major evolutionary transition occurred in the sarcopterygian fin, and that was the shift from a tribasic fin to a monobasic fin, with one basal pterygiophore (mono, meaning “one”). Recall that Sarcopterygii includes tetrapods and the other lobe-finned fishes. In the lineage leading to tetrapods, the fin develops along a distinct axis of elements that runs the posterior length of the fin. As a result, the radial pterygiophores only emerge on the anterior side of this axis. This particular fin configuration is called a crossopterygian fin, and if we look at the elements of the crossopterygian fin, we can see clear homologies with the elements of the tetrapod limb (Figure 10.6).
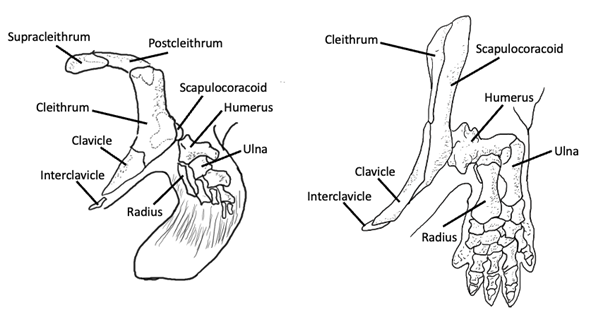
Figure 10.6—Sarcopterygian appendages: crossopterygian fin (left) and tetrapod limb (right). Notice the single basal pterygiophore and homologous structures in both appendages. Adapted from Liem et al., 2001.
Before delving into the details of the tetrapod limb structure, you should be familiar with the terminology for the sections of the typical tetrapod limb. The standard tetrapod limb has three distinct sections. From proximal to distal, they are as follows:
- Stylopodium: This is the brachium (upper arm) of the forelimb and the thigh of the hindlimb.
- Zeugopodium: This is the middle section of the limb and includes the antebrachium (radius and ulna) of the forelimb and the crus (or “shin”; tibia and fibula) of the hindlimb.
- Autopodium: This is the manus (hand) and the pes (foot), which are made up of many small bones. These include
a.carpals and tarsals, the small bones that compose the wrist and ankle, respectively;
b.metacarpals and metatarsals, which give structure to the palm of the hand and the sole of the foot, respectively; and
c.phalanges, which make up the digits (fingers and toes).
In Figure 10.7, the fossil sarcopterygian fish is on the left, and the basal tetrapod is on the right. If you follow along the posterior margin, the axis of pterygiophores is shown with the red line, and you’ll see the radials emerging anteriorly. You can also see that in both appendages, there’s a single proximal element, followed by two distal elements. However, if we look at the distal area, there’s a greater number of distal elements in tetrapods compared to the fossil sarcopterygian. An important point to recognize about the distal elements of the sarcopterygian is that they cannot just be modified fin rays, because the lepidotrichia that make up the fin rays are formed from dermal bone (meaning that they form through the process of intramembranous ossification). However, the digits of tetrapods are endochondral in origin, meaning they are formed through a different ossification process, thus indicating a distinct origin from the lepidotrichia of sarcopterygian fins.
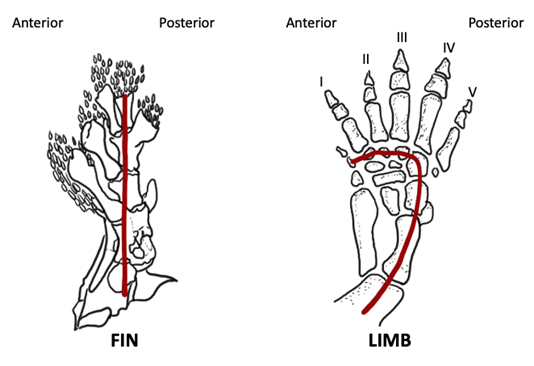
Figure 10.7—Fossil sarcopterygian fin (left) and tetrapod limb (right). The developmental axis of the fin/limb is indicated by the red line running the length of the posterior side. Adapted from Liem et al., 2001.
As we begin to examine the limbs of tetrapods, you’ll notice that the structure of the forelimbs and hindlimbs is very similar, and we typically describe limb structure of both appendages using a series of proximal to distal sections (i.e., stylopod, zeugopod, and autopod). One additional item to be aware of regarding the tetrapod limb, however, is the numbering schemes used to define the phalanges of the autopodium. In fact, there are two numbering schemes you should be aware of:
- The first number scheme assigns each digit a number, starting from what was ancestrally anterior and moving to posterior. So in humans, the thumb and big toe (hallux) are each termed “Digit 1,” and the pinky finger and little toe are defined as “Digit 5,” with the intermediate fingers/toes numbered in order (i.e., index finger = Digit 2, middle finger = Digit 3, ring finger = Digit 4).
- The second numbering scheme is what is called the phalangeal formula. This formula lists the number of phalanges in each digit in order from Digit 1 to Digit 5; ancestrally, the phalangeal formula was 2-3-4-5-3, meaning that Digit 1 was composed of two phalangeal bones, Digit 2 had three phalangeal bones, Digit 3 had four, and so on.
Recall that the axis of element formation runs along the posterior side of the tetrapod limb (Figure 10.7), and as a result, it was originally thought that this axis extended through the digit that was the longest (ancestrally, Digit 4). This would have meant that the anterior digits were simply extensions of the radial pterygiophores, and only Digit 5 was new, given its posterior position to Digit 4. However, more recent developmental studies have actually shown that the sequence of digit formation turns along the distal edge of the hand or foot (Figure 10.6, notice the curve in the axis); thus all the digits are actually on the opposite side of the axis from the radials and therefore must be novel structures. This pattern also gives us a mechanism for understanding digit reduction: If development along the axis was truncated or terminated early, fewer digits would form!
10.4 Evolution
Early Tetrapods
Early tetrapods tended to have big, blocky bones with large processes for muscle attachments. Nonetheless, the earliest limbs were relatively small given the size of the organism (Figure 10.8).
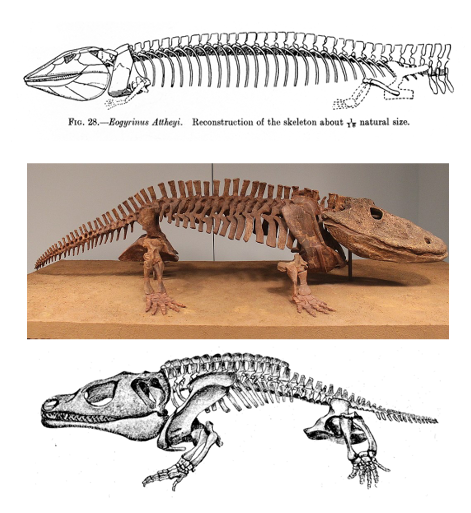
Figure 10.8—Examples of early tetrapods (Eogyrinus, top; Eryops, center; Cacops, bottom). Notice the relative size of the limbs compared to the body and the blocky bone structure of the appendages, particularly in Eryops and Cacops.
The blockier bones and large sites for muscle attachment help support the body weight of the animal. In the early tetrapods’ pectoral girdle, there was a single scapulocoracoid ossification, with the scapula positioned dorsally and coracoid ventrally. The pectoral girdle also had an articular facet for the humeral head, called the glenoid (Figure 10.9). However, these animals had reduced dermal elements in the pectoral girdle compared to fishes. In fact, in tetrapods, only the cleithrum, clavicle, and interclavicle remain.
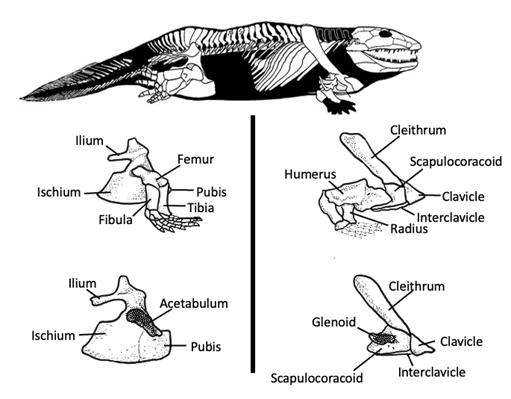
Figure 10.9—Pectoral and pelvic girdles in early tetrapods (Ichthyostega).
What’s more, by this point in the evolutionary timeline, the pectoral girdle was detached from the head (a change that occurred in Devonian tetrapodomorphs, approximately 400 MYA), thereby creating a distinct neck and resulting in a pectoral girdle attached to the body axis only by the presence of a muscular sling (Figure 10.10).
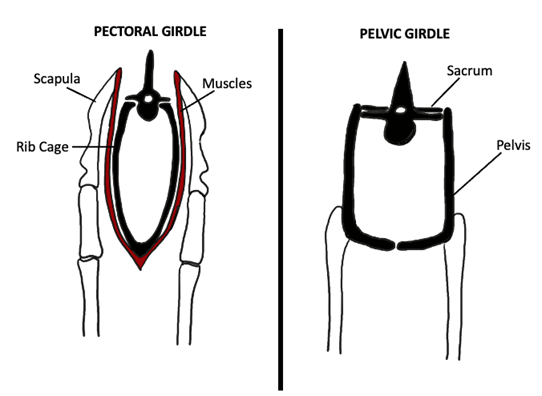
Figure 10.10—Attachment of the pectoral (left) and pelvic (right) girdles to the tetrapod trunk. Notice that the pectoral girdle is attached only by the presence of a muscular sling. Adapted from Liem et al., 2001.
In contrast to the pectoral girdle, the regions of the pelvic girdle were produced from distinct bones (although these fuse during development to form a fairly unified pelvic structure). The three bones that contribute to the formation of the pelvis are the ilium (dorsally), pubis (anteriorly), and ischium (posteriorly; Figure 10.11). All three of these bones contribute to the formation of the acetabulum, better known as the hip socket. Furthermore, the left and right pelves fuse along a midline juncture called the pelvic or pubic symphysis, thus uniting the left and right pelves (Figure 10.12).
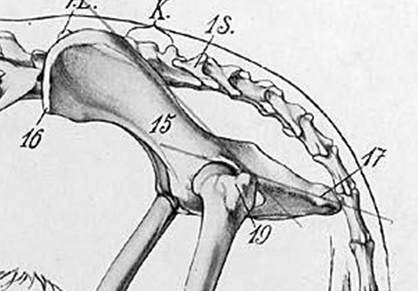
Figure 10.11—The pelvis of a dog: 15 is the ilium, 17 the ischium, and the region bordered by 19 is the pubis.
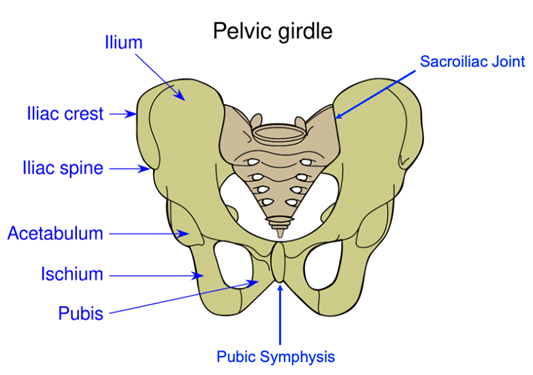
Figure 10.12—Anatomy of the human pelvis. Notice the pubic symphysis, uniting the left and right pelves.
Remember, as we saw in Chapter 3, based on fossil evidence, these girdle and limb features started to appear in animals with big tail fins. What this tells us is that weight-bearing limbs started to evolve in primarily aquatic animals. So although these structures allowed for locomotion on land, it is likely that they originally helped animals muck around in vegetation-choked shallow-water habitats.
Derived Tetrapods
The blocky structure of the limbs in the first tetrapods kept them low to the ground. A number of later modifications appear and result in limb elongation, elevation of the body off the ground, and modifications of lever mechanics (through the elaborations of processes on the bones onto which muscles attach). One such early modification occurs in the proximal ulna. Many animals show extensions of the ulna posterior to the elbow joint; this posterior process is called the olecranon (Figure 10.13). The lengthening of the olecranon increases the mechanical advantage of the muscles that extend the elbow, which then amplifies the force the limb produces. Recall from Chapter 5 that Fin × Lin = Fout × Lout, and if you increase Lin and keep Fin the same, you increase Fout. Increasing the length of the ulna posterior to the joint in this case increases Lin and allows a substantial increase in the force coming out of the system.
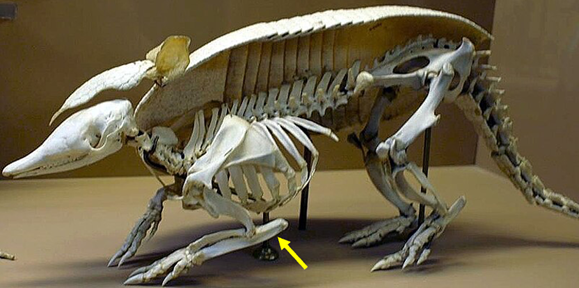
Figure 10.13—Armadillo forelimb showing huge expansion of the olecranon posterior to the elbow joint (olecranon indicated by yellow arrow).
Amniotes
Amniotes show consolidation of several tarsals into a single structure called the astragalus in the ankle (Figure 10.14). The astragalus (also known as the talus) serves as a key articulation point between the tibia and fibula of the lower leg and the bones of the foot. This novel structure provides stability to the ankle joint while also allowing a wide range of motions.
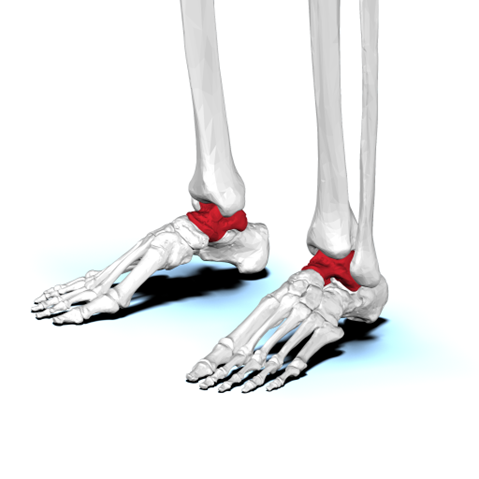
Figure 10.14—Condensation of the tarsals form the astragalus bone (a.k.a. talus; indicated in red) in the tetrapod ankle, shown here in a human.
In addition, amniotes and other derived tetrapods show several changes to the structures of both the pectoral and pelvic girdles:
- In the pectoral girdle, the tendency is to reduce dermal elements. All derived tetrapods actually lose the cleithrum, and some also lose the clavicle and interclavicle. Furthermore, the scapula and coracoid condense separately in derived tetrapods and amniotes. These changes to the pectoral girdle facilitate increased range of motion in the forelimb.
- In the pelvic girdle, a foramen develops between the pubis and ischium in many taxa; this foramen expands the margins of the bones, thereby increasing available surface area for muscle attachment. Several lineages also show changes to the pelvic girdle that are related to specific locomotor methods. For example, frogs have a huge anterior projection on the ilium, which functions to increase the surface area for attachment of the muscles that power jumping.
There are also several lineages that show distinctive changes to the structures of the autopodia; these changes are typically related to locomotion, especially swimming and flying. For example, ichthyosaurs form paddles from their forelimbs by radically increasing both the number of digits (called polydactyly) and the number of phalanges present in each digit (called polyphalangy; Figure 10.15).
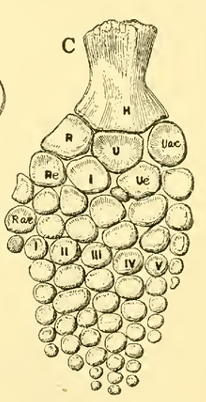
Figure 10.15—Ichthyosaur paddle. Notice the increase in both the number of digits and the number of phalanges per digit in the autopodium.
Flight also involves a number of substantial modifications to the appendicular skeleton. Three different vertebrate lineages independently evolved flight, and they each evolved a different way to construct a wing (Figure 10.16):
- Bats elongate all the digits of the hand.
- Pterosaurs only elongate Digit 4 and shorten proximal bones of the arm.
- Birds actually lose two digits on the hand and fuse the carpals and metacarpals into a single structure called the carpometacarpus (Figure 10.17).
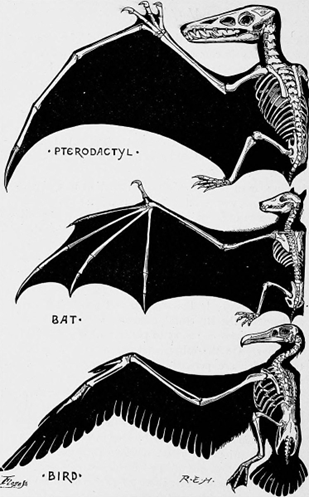
Figure 10.16—Three different wing configurations in the taxa that evolved powered flight: pterosaurs (top), bats (center), and birds (bottom).
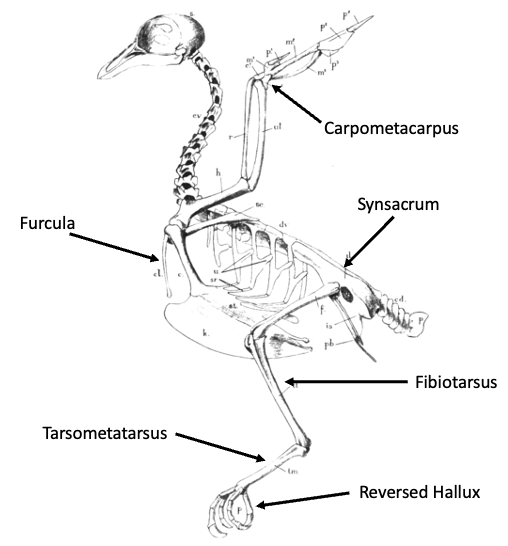
Figure 10.17—Bird skeletal anatomy. Notice the modifications to both the forelimb and hindlimb structures in this group.
Aves
In addition to the changes to the hand, another forelimb modification unique to birds is the fusion of the left and right clavicles to form a structure called the furcula. The downstroke of the wings pulls the clavicular structures apart, but the furcula acts like a spring and recoils when the muscular tension from the wing downstroke is released, thus helping pull the wings back up for the upstroke and resulting in an energy savings (because less muscular action is needed for the upstroke portion of the wingbeat).
Even though flight is a forelimb-powered activity, appendicular modifications in birds are not just restricted to the forelimb. Indeed, bird hindlimbs also show a number of modifications (Figure 10.17):
- One of these is the formation of the synsacrum in the pelvic region; this structure is partly formed by a posterior rotation of the pubis that helps consolidate the pelvic girdle.
- Bird hindlimbs are oriented more directly under the body (known as a parasagittal limb posture), a posture shared with their cursorial theropod relatives, rather than sprawling out to the side as observed in other reptiles (such as lizards or crocodylians). Bird legs also tend to have a “crouched” position, which functions to reposition the body’s center of mass to counterbalance the mass of the large flight muscles.
- The fibula is substantially reduced in birds.
- The tarsals consolidate onto both the proximal hindlimb element (forming the fibiotarsus) and distal element (forming the tarsometatarsus).
- Finally, Digit 1 (“hallux”) of the pes of birds rotates posteriorly, thus providing an opposable digit that facilitates grasping and perching.
Mammals
Similar to birds, one major trend in appendicular modifications observed in mammals is that the limbs are brought under the body to produce a more upright posture. And as expected, a number of changes in the limbs and girdles are related to this change in posture. First, there is a further reduction of dermal elements in the pectoral girdle of mammals, so much so that only the clavicle remains in the therian mammals (Figure 10.18).
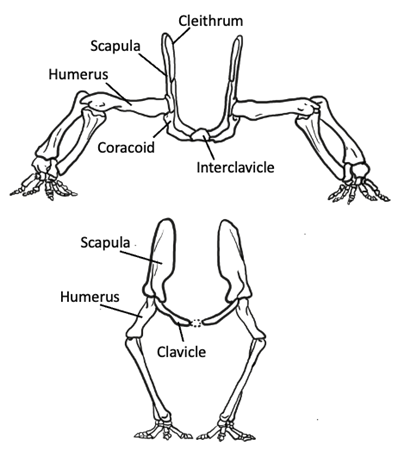
Figure 10.18—Pectoral girdle of ancestral synapsid (top) versus mammal (bottom). Notice the change in limb posture and accompanying reduction of the ventral girdle elements. Adapted from Liem et al., 2001.
In fact, many mammals reduce or lose the clavicle as well, such that the pectoral girdle is only connected to the axial skeleton via muscles and tendons. Because this upright posture requires far less ventral musculature, the necessity of skeletal support and structure in that region is less; therefore, it is not surprising that mammals exhibit a reduction in the ventral part of the limb girdle. Ancestrally, nonmammalian synapsids had two coracoid bones in the ventral girdle, but in therian mammals, the anterior coracoid is lost and the posterior coracoid is reduced to a tiny process on the scapula, anterior to the glenoid. As a result of this reduction in the pectoral girdle, the glenoid now faces ventrally rather than laterally, thus directing the humerus downward instead of sideways (Figure 10.18). Furthermore, along with this reduction of the ventral girdle, the dorsal muscles of the shoulder are elaborated. This muscle elaboration is accompanied by a dorsal expansion of the scapula, such that the old cranial border of the scapula actually becomes elevated and forms the scapular spine with a supraspinous expansion dorsocranial to it; these changes provide additional surface area for muscle attachment (Figure 10.19). These changes to the pectoral skeleton correspond with changes in the musculature, which exhibits a similar ventral reduction and dorsal elaboration, reflecting the need for increased mechanical support of the shoulder joint as the forelimb swings through longer arcs during locomotion.
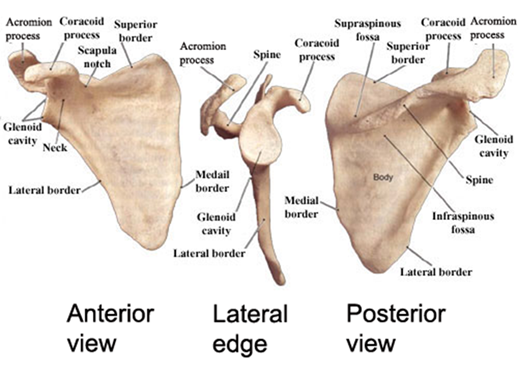
Figure 10.19—Human scapula anatomy. Notice the small projection anterior to the glenoid (coracoid process) as well as the dorsal expansion of the scapula to form the scapular spine and supraspinous fossa.
There are also a number of changes to the pelvic girdle. The pelves become elaborated dorsally and reduced ventrally in mammals, with a particularly noticeable expansion of the central obturator foramen, which was just a small hole that allowed passage for a nerve in the ancestral condition. One major difference in the modifications to the pectoral versus pelvic girdle, though, is the orientation of the joint socket. Recall that in the pectoral girdle, the glenoid becomes oriented downward as ventral elements of the girdle are reduced; however, the acetabulum of the pelvic girdle remains laterally oriented. This means that in order for the femur to be directed downward, the femur’s articular head now has to be reoriented to face laterally so it can articulate with the hip (Figure 10.20).
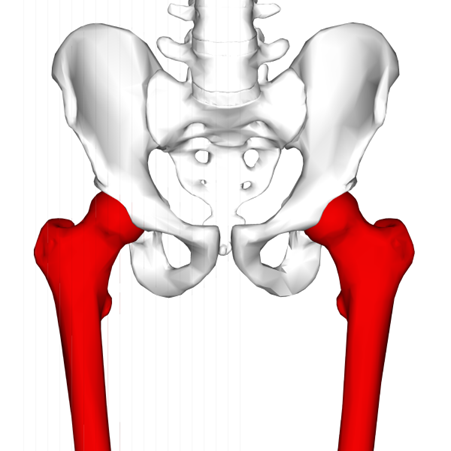
Figure 10.20—Human femur (red) and pelvis. Notice the lateral orientation of the femoral head to allow articulation with the acetabulum.
In the distal limbs of mammals, the radius and tibia become the primary weight-bearing bones, but the elbow points backward in mammals. This means that for the digits of the hand to point forward, the radius has to rotate about the distal end of the ulna. This rotation is called pronation, and you can actually do this with your own arm! The opposite motion is called supination. If you grip your forearm and rotate your wrist such that the palm is oriented downward, you can feel your radius pivot and cross over your ulna (pronation). Rotate your palm the other direction so that it faces up (supination), and you can feel your radius cross back over the ulna so that the two bones now lie parallel to one another (Figure 10.21).
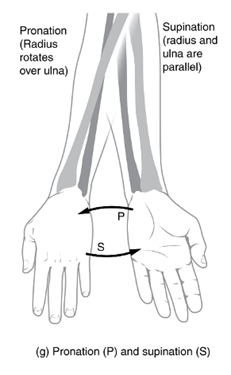
Figure 10.21—Pronation and supination of the forearm. Notice how the radius pivots to cross over the ulna during pronation.
In contrast, the knee points forward and acts like a simple hinge. One feature of this hinge in mammals is that it often includes an extra, non-weight-bearing bone. This type of bone is called a sesamoid bone, and the particular one found in the knee is called the patella; you may know it better as the “kneecap.” If we look at the knee in lateral view (Figure 10.22), you’ll see that what the patella does is increase the moment arm of the muscles that extend the knee. In essence, the patella acts like the olecranon of the elbow to increase mechanical advantage and amplify muscle-force output of the knee joint.
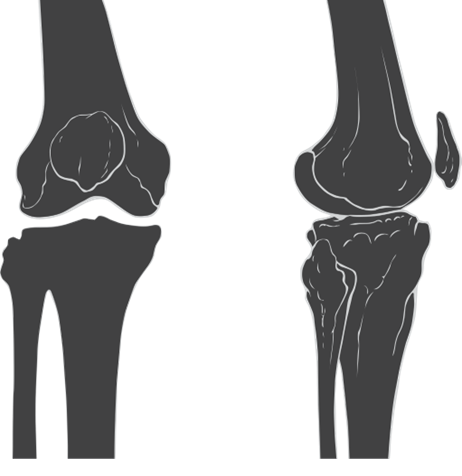
Figure 10.22—Anterior (left) and lateral (right) illustrations of the human knee. Notice the non-weight-bearing patella bone “floating” in front of the distal femur. This bone is actually embedded in the patellar tendon, a strap of connective tissue that spans the front of the knee joint.
Like reptiles, mammals also have an astragalus in the ankle (called the talus in humans), and one of the tarsal bones, the calcaneus, develops a posterior process called a tuber. The calcaneal tuber functions similarly to the olecranon to increase the mechanical advantage of the muscles that extend the ankle. Interestingly, the calcaneal tuber is not unique to mammals; it is also seen in crocodilian ankles.
Box 10.1—Fins to Limbs to Flippers
The invasion of land by early tetrapods and the evolution of tetrapod limbs from fins have long been considered to be among the landmark events in vertebrate evolution. However, a number of terrestrial vertebrate lineages secondarily returned to aquatic habitats (e.g., whales, mosasaurs, penguins, sea turtles). These secondarily aquatic transitions represent a number of independent returns to the water but are nonetheless characterized by near-universal morphological changes to the appendicular skeleton. Most notably (but not exclusively) for the purposes of this chapter is the evolution of flattened, flipper-like limbs in these taxa. As we know from our study of the relationship between form and function thus far, morphology is often driven—at least in part—by the functional demands of the environment in which an organism lives. But how does one get to a flipper from the typical tubular tetrapod limb?
One likely driver of flipper evolution can be found in differences in limb bone loading between terrestrial and aquatic environments. During terrestrial locomotion, load magnitude is higher due to the lack of environmental body support on land (i.e., air doesn’t help support the body the same way water does). In sprawling taxa, like turtles, there’s also a substantial difference in the orientation of limb bone loads during walking compared to swimming, with higher degrees of twisting occurring in the bone on land. Because round cross-sectional shapes resist these twisting loads more effectively than flattened cross-sectional shapes do, these loading regimes likely dictate tubular limb bone shapes on land. However, because twisting loads are reduced during swimming, animals may be released from this functional demand in water, thereby opening the door for the evolution of flattened shapes that confer hydrodynamic advantages.
Phylogenetic history and ancestral swimming style may also contribute to morphological evolution and secondary aquatic transitions. Recent work by Kiersten Formoso and colleagues broadly examined secondarily aquatic taxa from both reptile and mammal clades. These different groups would have swum very differently, showing radically different degrees of axial (primarily reptiles) versus appendicular (mammals and birds) propulsion. These clades also exhibit a number of preexisting morphological differences that evolved on land, including differences in posture (sprawling vs. upright) and variations in the structure of the limb elements (consider bird appendicular morphology, described in Section 10.4 of this chapter). As such, it is likely that ancestral terrestrial locomotor methods and morphology influenced swimming style in secondarily aquatic taxa, which by extension could influence aquatic morphology and flipper evolution. Further work in this area is still needed in order to understand the precise drivers of flipper evolution, but this is an exciting area of current research that requires an integrative approach, bringing together principles of evolution, ecology, development, and biomechanics, among others.
10.5 Summary
Fins and limbs are arguably some of the most fascinating and well-studied structures of the vertebrate body, likely due to their wide variation in structure and relationship to locomotion. The appendages evolved initially as stabilizing structures for aquatic swimming and then diversified to produce structures equipped for locomotion in a range of aquatic, terrestrial, and aerial habitats. Nonetheless, homologies across taxa in the appendicular skeleton point to a common evolutionary origin in early fishes.
Application Questions
- Once the major limb bones evolve in tetrapods, we see very few instances where those bones are lost. What functional hypotheses could you consider for this conservation in bone number?
- There are many instances where limb bones fuse together (wings of a bird, distal limb of a horse). What advantages do these fusions provide for the vertebrates in question?
10.6 Further Reading
- Bayramov, Andrey V., Sergey A. Yastrebov, Dmitry N. Mednikov, Karina R. Araslanova, Galina V. Ermakova, and Andrey G. Zaraisky. “Paired fins in vertebrate evolution and ontogeny.” Evolution and Development 26, no. 3 (2024): e12478.
- Begemann, Gerritt. “A debunked hypothesis resurrected: Shared developmental programs between paired limbs and vertebrate gill arches.” Zebrafish 6, no. 3 (2009): 303–304.
- Formoso, Kiersten K., Habib, Michael B., and Jorge Vélez-Juarbe. “The role of locomotory ancestry on secondarily aquatic transitions.” Integrative and Comparative Biology 63, no. 6 (2023): 1140–1153.
10.7 References
- Amerman, Erin C. Human Anatomy and Physiology, 2nd ed. New York: Pearson Education, Inc., 2019.
- Ashley-Ross, Miriam, et al. “Vertebrate land invasions—past, present, and future: An introduction to the symposium.” Integrative and Comparative Biology 53, no 2 (2013): 192–196.
- Bayramov, Andrey V., Sergey A. Yastrebov, Dmitry N. Mednikov, Karina R. Araslanova, Galina V. Ermakova, and Andrey G. Zaraisky. “Paired fins in vertebrate evolution and ontogeny.” Evolution and Development 26, no. 3 (2024): e12478.
- Begemann, Gerritt. “A debunked hypothesis resurrected: Shared developmental programs between paired limbs and vertebrate gill arches.” Zebrafish 6, no. 3 (2009): 303–304.
- Blob, Richard W., Christopher J. Mayerl, Angela RV Rivera, Gabriel Rivera, and Vanessa K Hilliard Young. “‘On the fence’ versus ‘all in’: Insights from turtles for the evolution of aquatic locomotor specializations and habitat transitions in tetrapod vertebrates.” Integrative and Comparative Biology 56, no. 6 (2016): 1310–1322.
- Brazeau, Martin D., Marco Castiello, Amin El Fassi El Fehri, Louis Hamilton, Alexander O. Ivanov, Zerina Johanson, and Matt Friedman. “Fossil evidence for a pharyngeal origin of the vertebrate pectoral girdle.” Nature 623, no. 7987 (2023): 550–554.
- Coates, M. I., and Jennifer A. Clack. “Polydactyly in the earliest known tetrapod limbs.” Nature 347, no. 6288 (1990): 66–69.
- Diogo, Rui. “Cranial or postcranial—Dual origin of the pectoral appendage of vertebrates combining the fin-fold and gill-arch theories?” Developmental Dynamics 249, no. 10 (2020): 1182–1200.
- Formoso, Kiersten K., Habib, Michael B., and Jorge Vélez-Juarbe. “The role of locomotory ancestry on secondarily aquatic transitions.” Integrative and Comparative Biology 63, no. 6 (2023): 1140–1153.
- Kardong, Kenneth V. Vertebrates: Comparative Anatomy, Function, Evolution, 8th ed. New York, NY: McGraw Hill Education, 2015.
- Liem, Karl F., William E. Bemis, Warren F. Walker, and Lance Grande. Functional Anatomy of the Vertebrates: An Evolutionary Perspective, 3rd ed. Belmont, CA: Brooks/Cole—Thomson Learning, Inc., 2001.
- McKinley, Michael P., Valerie D. O’Loughlin, and Elizabeth E. Pennefather-O’Brien. Human Anatomy, 5th ed. New York: McGraw Hill Education, 2015.
- Nursall, J. R. “Swimming and the origin of paired appendages.” American Zoologist (1962): 127–141.
- Tzung, Keh-Weei, Robert L. Lalonde, Karin D. Prummel, Harsha Mahabaleshwar, Hannah R. Moran, Jan Stundl, Amanda N. Cass et al. “A median fin derived from the lateral plate mesoderm and the origin of paired fins.” Nature 618, no. 7965 (2023): 543–549.
- Young, Vanessa K Hilliard, and Richard W. Blob. “Limb bone loading in swimming turtles: Changes in loading facilitate transitions from tubular to flipper-shaped limbs during aquatic invasions.” Biology Letters 11 (2015): 20150110.
- Young, Vanessa K Hilliard, Starner, Mary Kate, Baeza, Antonio J., and Richard W. Blob. “Thinking inside the box: Comparative limb bone shape in emydid turtles.” Journal of Herpetology 55, no. 2: 112–118.
- Young, Vanessa K Hilliard, Wienands, Charlotte E., Wilburn, Brittany P., and Richard W. Blob. “Humeral loads during swimming and walking in turtles: Implications for morphological change during aquatic reinvasions.” Journal of Experimental Biology 220 (2017): 3873–3877.
- Zimmer, Carl. At the Water’s Edge: Fish with Fingers, Whales with Legs, and How Life Came Ashore but Then Went Back to Sea. Simon and Schuster, 2014.

