8 The Skull
Kassandra Ford and Bill Ryerson

Figure 8.1—The skull of the domestic dog, Canis lupus familiaris.
Focus Questions—to Guide Your Reading of This Chapter
- How is feeding important to the shape and structure of the skull?
- How does the transition from water to land drastically impact the workings and structure of the skull?
- What are the three major functions that skulls must undertake, and what trade-offs must be made to accomplish all these functions?
8.1 Introduction
Skulls are ubiquitous in science and popular culture. If you asked someone to draw a skeleton, most would start by drawing a skull, and likely a human skull (Figure 8.2).

Figure 8.2—The skull of an adult human, with major bones and structures labeled.
It is the single most recognizable piece of the skeleton. Beyond the draw of its shape, style, and macabre symbolism, skulls are undeniably important in the biology and evolution of vertebrates. Skulls are unique; they are one of the only places in a body where multiple systems converge, competing for space, and additional behavioral elements add complexity. The skull houses the brain, the major player of the central nervous system. The cranial nerves all travel through the skull, requiring holes (foramen/fissures) or canals to pass through. Four of the five major senses are also housed in the skull: smell, taste, vision, and hearing. Intake for the respiratory system occurs in the skull, whether it’s air for the lungs or water passing through the gills. Most importantly (according to one of the authors of this chapter), the skull plays an outsized role in feeding behavior. Teeth, jaws, braincase, orbits, arches, and fenestrae will all be important as we examine the evolution and function of the vertebrate skull. Skulls with weapons, extremely kinetic (movable) skulls, skulls with multiple jaws, skulls with too many teeth, skulls with no teeth, and even more amazing examples will dot this chapter. All these elements, however, cannot overlap perfectly. Instead, we will observe how the different senses, brain evolution, and feeding modalities compete with one another for space and how different species solve this competition.
8.2 General Structure of the Skull
Despite the incredible variety observed in skull structures, shapes, sizes, and functions, there are several key elements that unite skulls together. The purpose of this section is to introduce you to some terminology and structures that are common throughout vertebrates so that as we start to discuss function and evolution, the key components will be familiar to you. There are a few different ways to refer to the structure of the skull depending on your perspective: functionally, developmentally, and evolutionary. There will be some overlap in the regions and areas of a skull, each with its own defining characteristics. Let’s take a look at Figure 8.3, a lateral view of a human skull.

Figure 8.3—A lateral view of the human skull.
You can see that the skull is separated into two different regions, the braincase and facial bones. The braincase (one word in most examples) is composed of the bones that encase the brain, simple enough. In humans, the remaining bones are considered the facial bones. There is some variation here between humans and other vertebrates; not all bones in many animals will belong to either a braincase or facial bones. It will help us to define some other regions and elements, particularly as we march our way through evolutionary history.
Neurocranium/Chondrocranium
The most basic type of skull found in vertebrates is known as the neurocranium. The neurocranium contains only the elements that enclose the brain, except for the roof. Imagine an empty cardboard box—this is essentially what the neurocranium is. There is no space for eyes, ears, or anything beyond the brain itself. Hagfishes, those deep-sea denizens, have a neurocranium with the addition of a thin roof made of cartilage over the brain. However, very quickly in history, the skull develops additional structures that increase in complexity and require further defining. As the brain and skull grow in size, modifications to the skull include not only additional bones but also landmarks for three special senses: vision, olfaction (smelling), and hearing. In the skull, we call these accommodations “capsules,” and they can be remembered as the three O’s (optic, olfactory, otic). The addition of a robust roof on the skull and the three capsules is referred to as the chondrocranium. This is the type of skull we see in our sharks (Figure 8.4).

Figure 8.4—The skull of a shark, lateral view. The chondrocranium is yellow.
Notice that the jaws are not attached to the skull in the shark as they are in the human. When the jaws do attach to the rest of the skull, this is an important evolutionary transition. For now, the jaws are not “part” of the chondrocranium.
Splanchnocranium
The splanchnocranium includes several arches and their derivatives, most notably the jaws. These arches include the mandibular arch, hyoid arch, and branchial/gill arches (Figure 8.4). These anterior arches are thought to have evolved to support respiration (moving water across the gills to obtain oxygen) but have since evolved for feeding purposes. All the arches contain either cartilage or bone (that previously replaced cartilage) and sit in and around the pharynx. The mandibular arch, or the “jaws,” contains the palatoquadrate and the lower mandibular bones (either Meckel’s cartilage in chondrichthyans or the early-developed dentary system in bony fishes). Posteriorly sits the hyoid arch, which includes a series of bones that support the jaws, such as the basihyal, ceratohyal, and the hyomandibula. The hyomandibula typically connects the otic capsule to the palatoquadrate, suspending the jaws. In chondrichthyans, a structure known as the spiracle is found in between the mandibular and hyoid arches. The spiracle is a small opening from the mouth/pharynx to the external environment. Interestingly, its function is not very well known! Finally, the branchial (or gill) arches are the bony components that surround the gill slits and support respiratory functions. The majority of bony fishes and chondrichthyans have five branchial arches (with five corresponding gill pouches), but some chondrichthyans have evolved additional arches. Each branchial arch contains five bones, all of which form a “series” across the five arches: pharyngobranchials, epibranchials, ceratobranchials, hypobranchials, and basibranchials (Figure 8.4).
Dermatocranium
Key in protecting the cranium is the dermatocranium, made up of the surface bones that cover the chondrocranium, the splanchnocranium, and the important internal components of the head (Figure 8.5).

Figure 8.5—A generalized developing embryo, illustrating the developmental origin of the dermatocranium and where it develops in relation to the other elements of the skull. Here the term viscerocranium refers to the seven arches.
In some groups of vertebrates (e.g., the bowfin fish Amia), the dermatocranium sits on top of the cartilaginous neurocranium, while in most groups, that cartilage is replaced by the dermal bones. This includes bones like the maxilla, premaxilla, dentary, hyomandibula, and opercular series, among many others. There are generally considered seven “series” of bones: facial, orbital, temporal, vault, opercular, palatal, and lower jaw (Table 8.1).
Table 8.1—The bones of the skull and the series to which each belongs
| Braincase | Mandible | |||||
|
Facial |
Orbital |
Temporal |
Vault |
Opercular |
Palatal |
Lower jaw |
|
Premaxilla |
Lacrimal |
Intertemporal |
Frontal |
Preopercle |
Vomer |
Dentary |
|
Maxilla |
Prefrontal |
Supratemporal |
Parietal |
Opercle |
Palatine |
Splenials |
|
Nasals |
Postfrontal |
Tabular |
Postparietal |
Subopercle |
Ectopterygoid |
Angular |
|
|
Jugal |
Squamosal |
|
Interopercle |
Pterygoid |
Surangular |
|
|
|
Quadratojugal |
|
|
Paraspenoid |
Prearticular |
|
|
|
|
|
|
|
Coronoids |
Often vertebrates will have teeth on several bony components of their dermatocranium, including on the dentary and premaxilla and often on the palatal bones or parasphenoid. We will cover the diversity of dentition and its placement later in the chapter.
As you are reading all these names and seeing all the figures, it is easy to lose track of all the bones and their names. Instead, focus on the bigger patterns of size, shape, and fusion.
8.3 Development
The development of the vertebrate skull can be thought of in two different forms: the cells and their origins (e.g., neural crest cells) but also the processes by which the individual bones are formed. To understand the different origins and cells involved in the development of the skull, remember that there are three major components or parts to the vertebrate skull: the chondrocranium, splanchnocranium, and dermatocranium. The chondrocranium, as the name suggests, is formed by cartilage for at least some duration. This requires three different elements that we must consider: neural crest cells, notochord, and mesoderm. The neural tube becomes separate from the ectoderm (which will become epidermal) above it during neurulation, and the cells of the neural crest will derive from the neural fold regions near the conjunction of the neural and nonneural ectoderm. Some of the neural crest cells will form the beginning elements of the notochord. The notochord, at its anterior end, grows forward and flattens out, forming the base of the chondrocranium. Mesodermal sclerotomes (blocks of mesoderm-derived cells distinct from neural crest cells but homologous to those that form the vertebrae in the next chapter) come together to form the caudal portion of the braincase, wrapping around the developing nerve cord and forming the foramen magnum. This will eventually become the occipital bones.
The splanchnocranium (including the gill arches, not just the jaws) is also derived from neural crest cells. These neural crest cells are the same as those formed during neurulation, as we just described, but instead these migrate down the body wall during development. In the region of the pharynx, the neural crest cells cease their migration and start laying down the elements of the splanchnocranium.
The development of the dermatocranium can largely be explained by the development of dermal bone in the chapter on the integument (Chapter 6). Briefly, neural crest cells that stay in the vicinity of the integument differentiate into cells we call ectomesenchymal cells. Ecto- and mese- in the names should tell you that these cells can (and do) play a role in the integument and connective tissues in a body. Additionally, dermatomes that are mesodermal in origin will also contribute to the formation of the dermatocranium.
Regarding process, recall that the bones are formed through two different processes: intramembranous ossification and endochondral ossification (see Section 7.5, Bone Development and Growth). The larger, flatter bones of the skull (parietal, frontal, temporal) are formed via intramembranous ossification, while the bones with more intricate shapes (mandible, squamosal, zygomatic) are formed through endochondral ossification. There is a trickiness to some of this, as elements of the chondrocranium that would be replaced by bone in development actually disappear as the intramembranously formed dermatocranium sinks down beneath the skin.
8.4 Evolution of the Skull
Jawless Fishes
Hagfishes (Myxinoidea) lack true jaws and vertebrae; they do have a chondrocranium and a simplified, cartilaginous versions of branchial arches called visceral arches. Within their mouth opening, they have keratinous tooth plates that allow them to scrape and “bite” their prey without the complex jaw system of other craniates.
Lampreys (Petromyzontioidea) also lack true jaws but do have a simplified version of vertebrae protecting a notochord (Figure 8.6).

Figure 8.6—A longitudinal section of a lamprey, with its anterior end to the right. The chondrocranium has been highlighted in red and seven of the eight visceral arches highlighted in blue.
They do not have a dermal component of their cranial skeleton, instead just having a chondrocranium and eight visceral arches that, together, form a branchial basket. They have a characteristic jaw opening filled with keratinous teeth that allows them to latch onto their prey, scrape a wound, and suck the blood of the organism.
†Ostracoderms are an extinct group of jawless vertebrates with incredible armor; they are most known for dermal shields on their flattened heads (see Figure 3.17 for some example ostracoderms). They were thought to be bottom-dwellers; therefore, this dorsoventral shield provided protection from potential predators above and around them. Because they lacked jaws, feeding was likely based on suction feeding rather than biting or crushing. Rapidly opening/expanding the mouth and oral cavity (together known as the buccal cavity) creates pressure changes that pull water and food into the mouth.
Gnathostomes
As discussed in Chapter 3, most vertebrates, except for the cyclostomes (hagfishes and lampreys), are part of the group Gnathostoma (Greek: “gnathos” = jaw; “stoma” = mouth), indicating the important evolutionary adaptation of jaws. We will cover jaws in more detail in Section 8.5, but for now it is important to make the distinction between jawless and jawed vertebrates, the latter of which contain almost all vertebrate diversity. Let’s dive into that diversity now!
Fishes
Fossil Group of Fishes
Placoderms were a group of jawed, armored fishes that were present from the Silurian to the Devonian. They had thick dermal armor and a joint between their head and thorax, allowing for a decent amount of head and neck movement. One of the most significant finds in recent years indicates that dermal jaw bones (historically thought to have evolved in bony fishes) were present in placoderms and therefore much earlier in evolutionary time than originally thought. It also means that the next group we’ll discuss in this section (chondrichthyans) likely lost those dermal bones and retained cartilage instead of bone. It’s important to note that researchers are still teasing apart the phylogenetic relationships of these ancient lineages, meaning we may get additional answers about the evolution of jaws in the future.
Chondrichthyans
Chondrichthyes have almost no bone, as they lost that characteristic in favor of retaining cartilage. Using evidence from the fossil record, we now think that the switch from bone back to cartilage occurred sometime after the jawless fishes branched off from their common ancestor with the rest of the vertebrates. This puts the cartilaginous fishes closer to the bony fishes than the jawless fishes. Because of this loss of bone, they do not have a dermatocranium (the bony head covering most fishes possess) but instead just have a chondrocranium and splanchnocranium. They have expanded that chondrocranium to cover their brain, forming a simplified version of a braincase (Figure 8.7).

Figure 8.7—The skull of the spiny dogfish, Squalus acanthias. Anterior side is to the right. Coloring is the same as Figure 8.4, where gray is the chondrocranium, red is the mandibular arch, and blue is the hyoid arch.
Their jaws are formed by the palatoquadrate and Meckel’s cartilage (also referred to as the Meckelian element), which are typically suspended from the ceratohyal with a ligament (Figure 8.4). Chondrichthyans typically fall into two different ecological categories: those that capture prey with their jaws and those that use their jaws for crushing prey (holocephalans, including chimeras, along with some skates and rays). They also have five to seven gill openings, with their gills being separated by thin plates of tissue. In some chondrichthyans, the placement of the first gill opening is somewhat constricted by the ceratohyal and hyomandibula, often leading to it being called a spiracle.
Early Actinopterygians
There is an incredible amount of morphological diversity in Actinopterygii (ray-finned fishes), making it very difficult to describe overall “trends” across the group. Several important adaptations evolved in this lineage, however, leading to increased skull mobility and food-processing abilities.
While chondrichthyans don’t have a dermatocranium, most other jawed vertebrates do possess this bony head covering, and it is rather obvious when looking directly at a skull. When looking at the more basal actinopterygians (i.e., Amia), nearly the entire skull is covered in dermal bones, leaving very little of the chondrocranium or splanchnocranium visible without further examination (Figure 8.8).

Figure 8.8—The skull of the bowfin, Amia calva. The chondrocranium is in blue, and the components of the mandibular arch are in yellow. The dermatocranium is red.
There are some gaps in the dermal bone, primarily around the ventral and caudal sides of the skull, allowing for some (but minimal) movement. Movement of bones in the skull (with the exception of the jaws) is known as cranial kinesis. This emphasis on the dermatocranium is consistent with many primitive fishes; this offers a large degree of protection but does not do much to dramatically increase mobility. There are several bones in actinopterygians, including the opercular bones and the extrascapulars, that contribute to the overall protection of the chondrocranium and gills.
Actinopterygii is also where we start to see the diversification of morphologies that evolved for food processing. Gill rays, a type of gill raker, are seen for the first time in acanthodians, an extinct group of actinopterygians. These gill rays (eventually becoming gill rakers) were stiff, bony protrusions on the branchial arches. Their position and structure suggest that they were used for filter feeding, sifting the water for food before it passed through the gills.
Actinopterygians and Teleostei
Taking the evolution of cranial kinesis to the maximum is Teleostei, a more derived Actinopterygii group. This group retains the same bones as the more basal Actinopterygii but often reduces the size and restructures the articulations between them (compare Figure 8.8 with Figure 8.9).

Figure 8.9—The skull of the largemouth bass, Micropterus salmoides. The image links to the CT scan of this individual.
In teleosts, the premaxilla and maxilla are able to articulate with each other and with the neurocranium in teleosts, allowing for a high degree of rotation and expansive jaw opening. This isn’t possible due to the size and robustness of those bones in Amia, therefore reducing the overall gape of those early actinopterygian fishes. These adaptations, including slightly reduced dermal bones and differing articulations, lead to increased mobility and therefore the ability to use rapid suction feeding as a method of prey capture (we’ll talk more about this soon). It’s very likely the ability to suction feed has aided in the rapid diversification of Teleostei, which boasts nearly 30,000 species.
Sarcopterygians
Sister to Actinopterygii (ray-finned fishes) is Sarcopterygii (lobe-finned fishes). See Chapter 10 on the appendicular skeleton for additional information about “ray-finned” versus “lobe-finned.” Sarcopterygii contains coelacanths, lungfishes, and all tetrapods (terrestrial vertebrates), although it’s important to note that some “terrestrial vertebrates” have evolved to recolonize the oceans permanently (i.e., some members of Cetartiodactyla and Sirenia) or semipermanently (i.e., some members of Carnivora, Rodentia, Monotremata, Afrosoricida, Euliphotyphla, and Didelphimorphia). All sarcopterygians have evolved lungs (in some way), usually aiding in air breathing.
There are several bones in actinopterygians that were lost in sarcopterygians—namely, the opercular bones and the extrascapulars. The loss of “gills” in the sarcopterygian ancestors of the tetrapods meant those bones were no longer necessary to protect that integral section of the skull, leading to dermal bones fusing together. Sarcopterygians are also where we start to see fenestrae, or openings in the outer dermatocranium. As these platelike dermal bones fuse together, openings are necessary for our sensory capsules to transmit information from the organism’s surface to its nervous system.
Coelacanths
The first group of sarcopterygians that we’ll discuss is the coelacanths (Coelacanthiformes). Coelacanths were thought to have gone extinct approximately 66 million years ago but then were discovered off the coast of South Africa (living!) in 1938. They are considered “living fossils,” possibly giving scientists insights into more “primitive” phenotypes from closely related species that went extinct. These fishes have gills, allowing them to breathe underwater, along with a primitive “lung” that remains small and largely useless after development. Coelacanths, along with all other tetrapods, evolved from an ancestor that had the first functioning lung. Coelacanths are the only remaining sarcopterygian with an intracranial joint, a joint between the anterior and posterior parts of the neurocranium, allowing the neurocranium to bend. Although this characteristic is now only seen in coelacanths, it was present in fossil sarcopterygians, including now-extinct species of lungfishes.
Lungfishes
Lungfishes take their name literally and have true, functioning lungs for air breathing (all other groups after this also have lungs). They begin their lives with gills but become obligate air breathers as their lungs develop. They have autostylic jaw suspension (discussed in Section 8.5 on jaws), allowing them to crush hard prey in a food-processing method called durophagy. They have large tooth plates that are used for crushing prey items.
A Brief Note on Tetrapods
Once the tetrapods begin to move onto land, it is easy to consider the appendicular skeleton as the element of the skeleton that is forced to undergo the most change in the transition from water to land (see Chapter 5 for more!). However, the skull is also going to undergo some extreme changes. Two of these changes we can introduce now, as they will impact all the tetrapods. The first is the evolution of a brand-new structure that we call the tongue. The tongue in all nonmammalian tetrapods has a significant skeletal structure to it, and its evolution creates opportunities for vertebrates to rely on the tongue to play a role in feeding. The second has to do with the environment. Two of the most common types of feeding in fishes, suspension and suction feeding, are not available to the land-dwelling tetrapods (more on this later in this chapter). Both of those feeding styles rely on moving the relatively denser water (and the prey within it), but moving air is not energetically efficient for tetrapods. Instead, tetrapods will rely heavily on biting (using both jaws to grab and secure the prey) or tongue prehension (using the tongue to grab the prey in the environment). A consequence of this is that those amazing kinetic skulls of actinopterygian fishes will lose a lot of mobility (to create large volumes for suction feeding) and instead become much more robust (a result of different bones fusing together and becoming larger) to handle the larger forces associated with biting and chewing. The increased fusion of the bones in most tetrapods leaves us with often jagged lines known as “sutures.” The sutures reflect where the individual bones come together. Sutures can be used to age some vertebrates, as the fusions become harder to identify as individuals get older. Gradually, many individual bones fuse to the point where we can consider them one functional unit. You may find it helpful to refer to the relationships among these groups in Chapter 3.
Amphibians
The first tetrapods to emerge onto land were the amphibians, and our understanding of their skull evolution must begin with their immediate ancestors, many of whom were still predominantly aquatic. One of the most famous fossil finds of the last 100 years is that of Tiktaalik, an amphibian ancestor (“fishapod”) that still very much lived like a shallow-water fish. Tiktaalik is important because its appendicular skeleton shows clearly how the transition from fins to limbs might have occurred. However, that is not the only story that Tiktaalik has to tell. The skull of Tiktaalik, like that of several other stem tetrapods (e.g., Ichthyostega), also shows how all the wondrous elements of the fish skull come together to form that robust skull discussed previously (Figure 8.10).

Figure 8.10—The skull of Tiktaalik. The image links to the CT scan of this fossil.
Notice that the upper jaw is completely fused to the skull along its entire length, from maxilla to quadrate. The lower jaw is quite long and made of several different bones. Teeth on the lower jaw are carried only on the dentary bone. The skull appears to be completely fused together and therefore immobile (also known as akinetic), and to our best knowledge this is the case with Tiktaalik.
There is one additional bone to note in the amphibians that will play a larger role later on in reptiles, birds, and mammals. The bone is called the columella, a thin, long bone running from the quadrate to the braincase. This bone carries vibrations from the jaws to the sensory apparatus of the inner ear, with more on this in the sensory chapter (Chapter 20).
Extant amphibians (Lissamphibia) largely retain this robust, immobile skull of their ancestors. From the frogs to the salamanders, no evidence of a kinetic skull can be found. Not all the amphibians resemble the ancestral condition represented by Tiktaalik. Many of the amphibians have even further reduced the number of bones of the skull through loss or fusion, making way for larger eyes and jaw muscles (Figure 8.11).

Figure 8.11—Representative skulls of a few amphibians: (A) the frog Humerana humeralis; (B) the aquatic salamander Necturus beyeri; (C) the terrestrial slimy salamander ;Plethodon glutinosus. Anterior is toward the top of the image and posterior toward the bottom in all images.
The amphibians have a striking amount of variation in their tongue skeleton. The bones associated with the tongue typically end in the suffix -hyal and are derived from the hyoid arch in fishes. The tongue skeletons are extremely variable in the amphibians, from the tiny and seemingly irrelevant tongue skeletons found in the mudpuppy Necturus to that of the plethodontid salamander Hydromantes. Hydromantes, found in the mountains of California, have tongues that shoot out twice their body length in the blink of an eye. To support this tongue, the skeletal elements are highly modified to anchor the tongue and muscles. Some of these muscles are so large, they extend all the way down to the hip, and the skeletal elements go almost as far!
Box 8.1—Temporal Roof Evolution—Here’s the Weird Stuff That’s About to Happen
The dermatocranium of early tetrapods evolved rapidly, and many of the small bones seen in actinoptergyians were lost, resulting in larger, fused bones in the skull. The temporal roof (sometimes called the dermal roof) retained the overall pattern of bones, but the number of connections between them shifted (Figure 8.12). Remember that the opercular series and the extrascapulars were lost in the Sarcopterygii from the Actinopterygii.
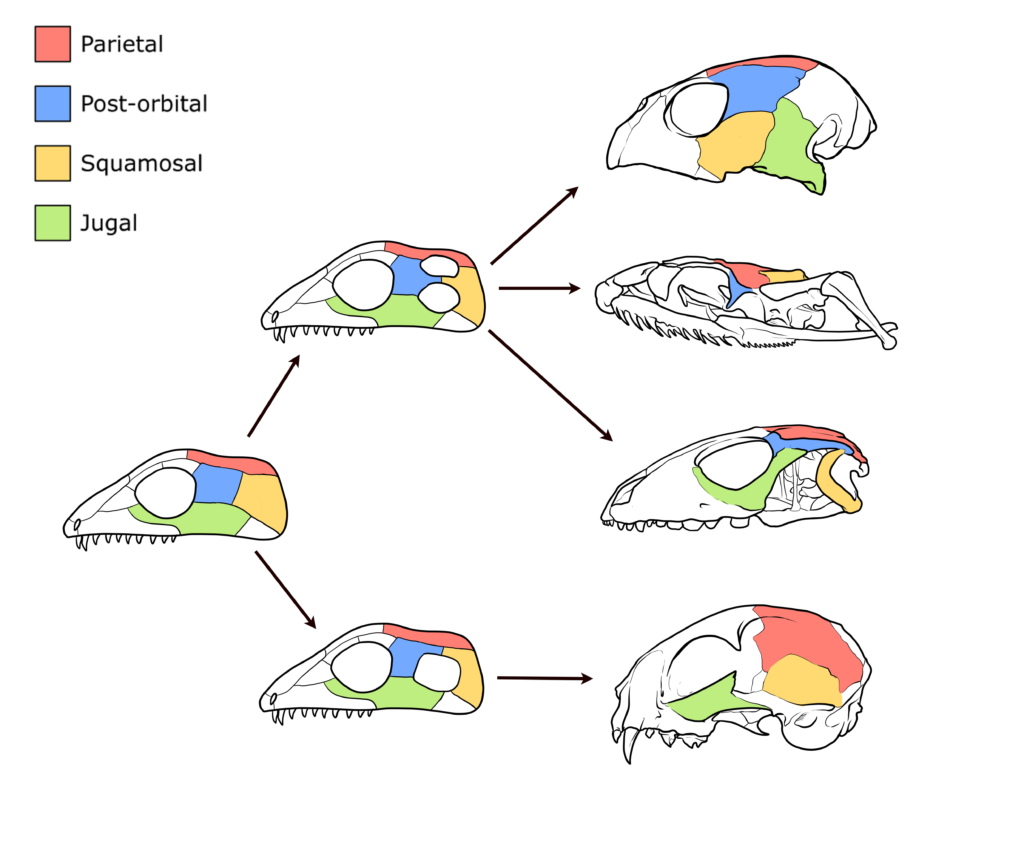
Figure 8.12—Evolutionary changes resulting in the fusion of certain cranial bones and the temporal roof found in tetrapods. The extant species on the far right are (from top to bottom): Green sea turtle (Chelonia mydas); North American rat snake (Pantherophis alleghaniensis); Caiman lizard (Dracaena guianensis); and domestic cat (Felis catus).
Instead of filling that space with expanded bones, most sarcopterygians shifted bones around, allowing for differences in the size and fusion of the skull bones.
Table 8.2—The bones that compose the temporal roof in tetrapods
|
Temporal roof of tetrapods |
||||
|
Lateral tooth-bearing series |
Circumorbital series |
Cheek series |
Median series |
Temporal series |
|
Premaxilla |
Septomaxilla |
Squamosal |
Nasal |
Intertemporal |
|
Maxilla |
Lacrimal |
Quadratojugal |
Frontal |
Supratemporal |
|
|
Prefrontal |
|
Parietal |
Tabular |
|
|
Jugal |
|
Postparietal |
|
|
|
Postfrontal |
|
|
|
|
|
Postorbital |
|
|
|
As we stated in the amphibians section, the dermal/temporal roof bones fuse together, becoming extremely resistant to the forces that happen during biting. This robustness comes at a cost. Earlier, we discussed that the robust skulls were limited in movement and that species with very robust skulls were not particularly kinetic. There is, however, a second issue that will arise. In the evolutionary time between the evolution of amphibians and the evolution of the common ancestor that gave rise to both reptiles and mammals, the temporal roof sank deeper below the skin while simultaneously the braincase had to enlarge as the brain changed in size. You might consider this to be a nonissue, as we are talking about bone running into bone. We are forgetting one key element and that is that there is muscle that is trapped between these two layers of bone. In particular, the jaw-closing muscles are the ones that are trapped between these two layers of bone. What’s a tetrapod to do? The answer seems to be to create small windows within the temporal roof, giving the jaw-closing muscles enough space to bulge during contraction. We call these windows temporal fenestrae, temporal referring to the region and fenestra being the Latin word for “window.”
Amphibians and the common ancestors to reptiles and mammals did not have any temporal fenestrae at all. We call this condition to be anapsid. The “apsid” refers back to the Greek “hapsis,” referring to an arch. The names in this case refer to not the windows but the arch of bone that is left behind. Anapsid therefore means “no arch.” From there, we find that there is a sharp distinction between the lineage that leads to the mammals and the one that leads to the reptiles and birds. Mammals belong to the clade that we refer to as synapsids, referring to the fact that they have one arch and one corresponding temporal fenestra. The Reptilia are diapsids, with two arches and two temporal fenestrae. When we discuss these lineages, we will rely on a few fossils as examples of what the fenestrae looked like. Note that many changes have occurred since the original evolution of these fenestrae, and many of the modifications are difficult to notice without knowing which lineage they belong to first. Groups like snakes (diapsids), turtles (technically diapsids), and even humans (synapsids) are tough to classify without knowing which group they belong to beforehand.
Reptilia (Diapsids)
Although the amphibian skull was not as altered after the transition to land, as we move onto the reptiles, we find that each major lineage of the reptiles (including the birds) alters the skull in their own way. The functional elements of these changes are varied, and some of them remain a mystery. A few of these (like the turtles) confused taxonomists for years, making their placement on the phylogenetic tree of vertebrates difficult.
To begin, let us approach the lizards and snakes. The common ancestor to all the lizards and snakes is a true diapsid, with both temporal fenestrae clearly visible. In modern lizards and snakes, we refer to the group as modified diapsids, where additional bone loss has made it seem as though there was only ever one temporal fenestra. There is one living example of a true diapsid, the enigmatic tuatara.
The tuatara, while resembling a lizard, is actually the closest living relative to the lizards and snakes. The two temporal fenestrae are quite clear on this “almost” lizard as the dashed lines in Figure 8.13B.

Figure 8.13—Representative skulls of nonavian reptiles from a lateral and dorsal view, with anterior toward the right and posterior toward the left. Species names link to CT scan data. (A) Savannah monitor, Varanus salvator; (B) Tuatara (with temporal fenestrae highlighted by dashed lines), Sphenodon punctatus; (C) Tropical rattlesnake, Crotalus durissus; (D) Loggerhead sea turtle, Caretta caretta; (E) Tyrannosaurus rex; (F) New Guinea crocodile, Crocodylus novaeguineae.
Beyond the temporal fenestrae, there is an extraordinary amount of variation and diversity in skull shape and structure (Figure 8.13).
One characteristic that reevolves from ancestors with more fused skulls in the lizards is cranial kinesis, that ability to move individual bones within the skull. There are different types of cranial kinesis in the reptiles, depending on where the movement occurs within the skull. Metakinesis refers to movement of the braincase relative to the rest of the skull. Mesokinesis (the most common type among lizards) is movement of the bones of the upper jaw and snout relative to the braincase. Finally, there is streptostyly, the movement of the quadrate independently of other bones. Different lizard groups exhibit differing levels of kinesis. Lizards like the Gila monster, with their large robust skulls, exhibit almost no kinesis, while the closely related monitor lizards exhibit varying degrees of mesokinesis. Several lizard groups also possess osteoderms, bony elements in their skulls that act as additional armor (but see the integument in Chapter 6 for more). The snakes are the masters of kinesis of the reptile world, with seemingly every bone in the skull being able to move independently. Snakes exhibit all three types of kinesis, and it seems that their extensive streptostyly is a key element in their ability to create an ultralarge gape and swallow prey many times larger than their head. Many species also have lost several of the bones in the skull, further decreasing their robustness but increasing their cranial kinesis. Compare the typical rattlesnake skull versus the skull of your local garter snake or water snake and you will see that the rattlesnake skull seems to be a skull missing several bones even when compared to other snakes, further illustrating the diversity of skull shape and structure even within groups.
The turtles are a fascinating group, for their skull as much as their shell. The first thing you may notice about the skulls of turtles is that they lack teeth (more on teeth later). The second thing you may notice is that in comparison to what we have been discussing in the other tetrapods, turtles do not have obvious temporal fenestrae. We refer to turtles as secondarily anapsid, having lost the temporal fenestrae during their evolution. The reasons for this remain unclear. It is possible that turtles required the extra armor around the head or that the food being eaten did not require the same level of musculature as in other tetrapods. Turtle skulls are quite robust, with large temporal roof bones and less obvious openings to the inner ear. With the additional robustness, turtle skulls are completely akinetic, lacking the cranial kinesis we previously discussed in the lizards and snakes.
The crocodylians are also characterized by extremely robust skulls. Alligators and crocodiles are flattened dorsoventrally, and this shifts where we consider structures like the temporal fenestrae. When we look at the alligator in comparison to the lizard, you can see how the change in the shape would shift the placement of several bones and other structures. As we saw in the turtles, alligator skulls are akinetic.
Dinosaurs (Diapsids)
Dinosaurs, as a whole group, have some of the most impressive skulls. These large, imposing skulls wow thousands of museum visitors every year. Now that we have a much firmer understanding of the structure and function of skulls, we see that dinosaurs are specialized diapsids, just like the other reptiles. Many of the structural changes we observe in the dinosaurs are by-products of their size as opposed to different functional demands resulting from dinosaur biology or ecology. In many ways, the smaller dinosaurs more closely resemble the lizards and birds, but we shall focus on one element of the larger dinosaurs that is worth examining. The skull of Tyrannosaurus rex (Figure 8.13E) is one of the most recognizable skulls in all the vertebrates. As you take a close look at the skull, you may notice something that jumps out at you. The skull of Tyrannosaurus, like many of the large dinosaurs, seems to have more openings/holes than those of other reptiles. We also refer to these openings as fenestrae, just as we did with the temporal fenestrae, and name them for their position in the skull (e.g., anterior fenestrae). The exact function of these fenestrae is unclear. One certain result is that the skulls of these dinosaurs would weigh far less, reducing the energy and muscle costs of lifting that heavy head around. Recently, it has been suggested that these areas may have held concentrations of blood vessels for two possible reasons. The first is that dinosaurs of this size are now thought to have been gigantotherms, their increased body size resulting in elevated body temperatures. These collections of vessels may have been useful to dump excess heat from the body, similar to the large ears of modern-day elephants. The second is that dinosaurs may have been sensitive to infrared radiation, and these areas of vessels may have resulted in hot spots of infrared radiation that served as signals to other dinosaurs nearby.
Birds (Diapsids)
As descendants of the reptiles, birds are also modified diapsids. Birds are extremely variable morphologically, and it should come as no surprise that the skulls of birds are just as variable. There are a few broad patterns that we can observe first and then highlight some of the unique variations. Much like we will see with mammals, there is an increase in the size of the braincase in birds. Like turtles, teeth have been lost, instead replaced with a keratin sheath that covers the upper and lower jaws. Beyond this point, bird skulls are extremely specialized on a species-level basis (Figure 8.14). This specialization is primarily diet related, but there are many reasons for certain anatomical specialties. Parrot skulls are very kinetic, and recently it has been shown that this kinesis allows them to use their beaks/jaws as a prehensile limb, helping them climb and locomote across branches. The northern gannet is an open-ocean seabird that can dive at high speeds into the water. These birds lack external nares, a prominent feature in all other tetrapods. To breathe, they must open their mouths. Peregrine falcons have small diverticula (dividers) in their nares so that as they dive at 200 miles per hour, the air rushing into their nares does not do any damage to the soft tissues. Owls have extremely large orbits to accommodate their eyes, which are also forward facing for added increased binocular focus. Finally, several woodpeckers have tongues (and tongue skeletons) that are long enough to actually wrap around the top of their head. Changes in bird skull and bill shape can occur quickly in response to changes in the environment and food availability. In fact, it was the beak of a group of birds known as Darwin’s finches that was used as strong evidence for how changes to the anatomy of organisms could occur.

Figure 8.14—Representative skulls of select bird species with anterior being toward the right. Species names link to CT scans. (A) Eurasian sparrowhawk, Accipiter nisus; (B) Tasmanian nativehen, Gallinula mortierii; (C) Kagu, Rhynochetos jubatus; (D) Shoebill, Balaeniceps rex; (E) Bee hummingbird, Mellisuga minima; (F) Green-winged teal, Anas crecca; (G) Ostrich, Struthio camelus; (H) Lesser yellow-headed vulture, Cathartes burrovianus; (I) Yellow-bellied sapsucker, Sphyrapicus varius; (J) American tree sparrow, Spizelloides arborea; (K) Red-tailed hawk, Buteo jamaicensis; (L) Northern gannet, Morus bassanus.
Synapsids
At the split between the ancestral reptiles and ancestral mammals, we used the temporal fenestrae as a structure that could help us distinguish between the two groups, with the diapsids having two and the synapsids having one. This is an important distinction, as the earliest synapsids resembled reptiles in many ways. The best example of this is the dinosaur-looking (but not a dinosaur!) pelycosaur Dimetrodon, famous for its large sail back. In many ways, Dimetrodon does closely resemble our image of a dinosaur: sprawled posture, scaly skin, and large blocky head. However, a look at the skull of Dimetrodon confirms it to be a synapsid, a distant ancestor of modern mammals (Figure 8.15).

Figure 8.15—A lateral view of the skull of Dimetrodon, a stem synapsid. The entirely black opening behind the orbit is the one temporal fenestra.
In many ways, we can use Dimetrodon as an example of a generalized synapsid, just as we used the tuatara as the example diapsid. While this section has focused on the evolution of the temporal fenestrae as one of the key characteristics that changed greatly during the tetrapod evolution, we will find that this is not the case as we discuss the mammals. At the time of Dimetrodon, there is not a lot beyond the temporal fenestrae to distinguish the synapsids from the diapsids.
As we move forward through evolutionary time, a few changes will occur that will additionally distinguish the basal synapsids from the reptiles. One of the most obvious changes is going to be a change in the shape and size of the braincase (Figure 8.16). As the size of the braincase increases, it distorts the other structures that we find useful for identification. Look back to Figure 8.2, the human skull. The large braincase distorts the location of the temporal fenestrae as well as all the facial bones relative to the basal synapsids. One of the earliest changes to appear is the change in the jaw joint. In all our vertebrates to this point, the quadrate (or structure homologous to the quadrate) articulated with the articular (convenient) to form the jaw joint. However, by the time we reach modern mammals, the articulation of the jaw joint is between the maxilla and the mandible. The transition from the basal jaw joint to extant mammals has several cascading impacts, including the evolution of the middle ear bones. The bones of both the upper and lower jaw that were once important for feeding were freed from this function and were co-opted for another very important function. We will explore this transition in more detail in Section 8.5 on the jaws (see Jaw Articulation and Middle Ear Bones), but know that all of this will occur in a series of stages between the basal synapsid Dimetrodon and more derived mammals such as the American black bear Ursus americanus. The other change that is going to occur in the evolution of more derived mammals from more basal mammals is more subtle but also has big implications. In mammals, a secondary or hard palate evolves between the nasal cavity and the mouth (Figure 8.17)—touch your tongue to the roof of your mouth, and you’ve found yours. The hard palate is derived from the aptly named palatine bones. The palatine bones each have a process that connects to the other, creating an empty channel lying above the mouth. The processes originally did not connect and acted to reinforce the upper jaw when chewing tougher prey. That toughness remains, with the added benefit of separating the airways from the mouth, allowing for breathing while feeding.

Figure 8.16—Representative skulls of living mammals. 3D models are linked in the species name. (A) Platypus, Ornithorhynchus anatinus; (B) Short-beaked echidna, Tachyglossus aculeatus; (C) Common wombat, Vombatus ursinus; (D) Black wallaroo, Osphranter bernardus; (E) Grizzly bear, Ursus arctos; (F) Fisher cat, Pekania pennanti; (G) Wolverine, Gulo gulo; (H) Tiger, Panthera tigris; (I) Chacma baboon, Papio ursinus; (J) Domestic horse, Equus caballus; (K) Orca, Orcinus orca; (L) Vampire bat, Desmodus rotundus.

Figure 8.17—The evolution of the hard palate in mammals. A lateral view is on the left and a ventral view on the right. Note the black openings on the ventral skull images (openings to the nasopharynx).
The monotremes, that earliest diverging group of the mammals, are often considered representatives of the ancestral condition for mammals. However, especially when it comes to the skull, it is important to remember that this group is highly derived in many ways, having had millions of years since they diverged from the rest of the mammals. Both groups of monotremes have lost their teeth, though toothlike structures are present in the juveniles before being lost (Figure 8.16). The separation between the orbit and the temporal fenestrae is gone, making it appear as though there were only one opening.
The marsupials closely resemble the placental mammals in skull features, shape, and size. As we observed in the birds, many of the differences between skulls are highly specific to individual species, so much so that it is possible to identify species without a complete skull. If presented with an unfamiliar mammal skull, how could you tell if it was a potential marsupial or placental? One distinguishing trait is found on the lower jaw. Near the jaw joint, on the rounded edge of the bottom of the jaw, is the angular process. In placental mammals, the angular process is vertical. Marsupials have an angular process that points medially (toward the midline). This distinctive process makes it much easier to differentiate between the two groups.
The placental mammals, like marsupials, are extremely variable from species to species and even group to group. One unique item that many terrestrial mammals possess is a collection of small, thin bones known as the nasal turbinates (or turbinate bones). These turbinate bones resemble thin, rolled-up scrolls of paper with lots of little holes punched in them. These bones play a role in the conditioning of air as it enters and leaves the nasal cavities. The thin bones have a large capillary supply within them. As air enters, it is warmed by the blood in capillaries, and its humidity increases before reaching the lungs. This prevents lung damage from inhaling cold, dry air. Conversely, as the air leaves the lungs, some of that heat and humidity can be recaptured. However, we can use different skeletal clues to inform us about what type of mammal we may be looking at. For example, within the order Carnivora, you can use the ratio of two lengths to tell the difference between a few different groups (Figure 8.16). In the family Mustelidae (skunks, weasels, mink, and fisher cats), the length of the skull from the center of the orbit to the back of the braincase is much greater than the length of the tip of the snout to the orbit. In the family Canidae (dogs, wolves, foxes), those two lengths are very similar. Beyond this, however, it is difficult to generalize across broad groups of mammals, as most differences tend to be very species specific, and there are always exceptions within groups. A good example of this is in the Rodentia, which includes mammals like mice, rats, beaver, and the capybara. Groups of rodents have similarly modified skulls that include differences in the infraorbital canal. Other groups have changes involving the teeth that can make them more easily identified. Some have lost their teeth (anteaters), while others have enlarged teeth that act as tusks from feeding and competition (walrus and elephants). Cetaceans (dolphins, porpoises, and whales) all have highly modified skulls, currently assumed to be the result of accommodating the aquatic lifestyle as well as amplifying their ability to echolocate. One group of whales (the mysticetes or baleen whales) has also lost their teeth, instead using keratinized sheaths (baleen) to filter shrimp and krill from the water.
The teeth of placental mammals are often a distinguishing character for individual species (more later on the importance and structure of teeth). In fact, some fossil mammals are only known from one tooth. Most mammals have teeth that are quite different from one another in certain places in the jaw, known as morphological heterodonty (more on this later). Broadly, most mammals have (in order from the front of the jaw to the back) incisors, canines, premolars, and molars. The number of each type of tooth, as well as its position, can be used to distinguish individual species of mammals. It is also worth noting that the number and type of teeth may also differ between the upper and lower jaws of a single species. To help us identify different species, we use what is called a dental formula to describe this pattern. In humans, for example, our dental formula is 2(I 2/2, C 1/1, P 2/2, M 3/3). The letter indicates the type of tooth (I for incisor), and the numbers indicate both location and number of that tooth type (2/2, two in the upper jaw and two in the lower jaw). The first “2” tells us that this represents one side (left/right), so if you want to count the total number of teeth in the mouth, you must multiply by 2. So now we can compare the human formula to that of a domestic dog: 2 (I3/I3, C1/C1, P4/P4, M2/M3). This is different from humans, and also note that there are more molars in a dog’s lower jaw than its upper jaw. While this gives us a tool for identifying different species, the hows and whats of this tooth identification are covered in a broader view later, in Section 8.6 on teeth.
Humans
It should come as no surprise that in many ways, the human skull is quite similar to other mammals. Humans have flattened facial bones and a large braincase, as do some closely related primates (Figure 8.2). There is one key difference in the skull of humans, as the shift to bipedalism resulted in changes to the orientation of the skull. The foramen magnum, through which the spinal cord leaves the skull, is now shifted ventrally. The orbits also change position, and we end up with two forward-facing eyes, like those seen in owls and cats, allowing for increased depth perception and binocular vision. Human teeth are similar to those of other mammals (more on teeth later). The major bones of the braincase—frontal, parietal, temporal, and occipital—are tightly fused together during adulthood. However, they are not completely fused together at the time of birth. The sutures that connect these various bones are still separated quite a bit by bands of connective tissue, leaving “soft spots” on a baby’s skull. These soft spots are technically named fontanels, and they will close shortly after birth. The current hypothesis is that these soft spots are retained until after birth to provide some level of flexibility during birth. The large braincase is difficult to pass through the birth canal, and the ability to slightly move the skull bones around can ease this process. However, this also means that the brain is not fully protected during these first crucial weeks after birth.
A Brief Note on the Following Section
To this point, we have discussed the generalized function of the skull, its development, and how evolutionary time imposed broad changes on the skull. However, given all we have said about the importance of the skull, it seems that we did emphasize many of these big changes. In this section, we focus on the jaws and how their evolution sheds light on not only the broader evolution of vertebrates but also how the functional demands of a changing environment forced vertebrates to adapt. We see this most clearly in the jaws of the vertebrates, which we will now explore in detail.
8.5 The Jaws
Hypotheses of Jaw Evolution
The evolutionary transition from jawless to jawed vertebrates was incredibly important to the diversification of vertebrates, but it is also a topic of debate among morphologists and developmental biologists. While the majority of those who study evolution adhere to the theory that jaws (the palatoquadrate, dentary) are modified gill arches, we are not sure about how or why it actually happened.
How?
Several lines of evidence are used to explain the primary theories of jaw evolution, including morphological examinations, developmental studies, and gene expression work. In gill-arch theory, scientists view the morphological resemblance between the jaw bones and the visceral/branchial arches as an argument that modifications were made to the anterior arch, resulting in jaws (Figure 8.18). Because the argument relies on bones that develop in series (the branchial arches and the argued derivatives), developmental studies examined sharks and saw similar developmental trends between the jaws and the visceral arches.

Figure 8.18—The three leading theories for the evolution of the jaws: serial, composite, and heterotopic. In serial gill-arch theory, the jaws are thought to evolve from the first two branchial arches. Composite gill-arch theory features losses and fusions of branchial arches, resulting in a complex jaw system. The heterotopic theory examined neural crest cells to determine the developmental beginnings of the jaw bones.
While this idea is (fairly) widely accepted, how those modifications were made is still up for debate. The main camps include serial theory, composite theory, and heterotopic theory; each of these is based on developmental and gene expression studies.
Serial theory posits that the mandibular arch was likely modified from either the first or second branchial arch and the hyoid arch from the following arch (some researchers believe the “first” branchial arch was lost, resulting in the second one forming the mandibular arch; Figure 8.18). This is based on the idea of serial homology and the similar development of those arches during the lifespan of an individual.
Composite theory is slightly more complex and also includes data from the fossil record. This theory doesn’t follow the “one arch, one mandible” view and instead includes a series of losses and fusions of the arches, resulting in the mandibular and hyoid arches (Figure 8.18). This work is largely based on morphological and developmental comparisons across extinct and extant taxa.
Gene expression work has turned some of these arguments on their heads. These studies, which lead to the heterotopic theory, are based on comparisons between larval lampreys and jawed vertebrates (Figure 8.18). There were significant differences in expression of the Hox gene in the mandibular and branchial arches across these groups, indicating there may be substantial differences in the developmental origins of these morphologies. This boils down to differences in the neural crest cell patterns across these lineages. Under this theory, the pattern of crest cells responsible for the upper lips in lampreys (Figure 8.18) is not exactly homologous to the pattern seen in the jaws of gnathostomes (Figure 8.18). This work primarily calls into question the genes ultimately responsible for the development of jaws, which may, in turn, change our understanding of how jaws first evolved.
Why Jaws?
Some arguments say that these modifications were to improve feeding, while others cite improved ventilation. Changing the most anterior arch into a feeding apparatus would help an organism open its mouth and obtain food. Alternatively, the ability to open their mouth could allow aquatic organisms to bring larger amounts of water past the gills, aiding in breathing and acquiring oxygen. Regardless of which purpose came first, the other advantage was likely realized very quickly.
Jaw Suspension and Attachment
While evolving physical jaws was a huge transition for vertebrates, these jaws (and their relevant bones) exist in the context of the entirety of the skull and must move in ways that are (theoretically) efficient for the functions of the organism. This movement is achieved in several ways (through muscles and ligaments), but the bony connections (or articulations) have important impacts on how the jaws are nested in the skull. This idea, called jaw suspension, describes how the jaws are anchored (or not) onto the chondrocranium.
Types of Jaw Suspension
Primitive Autostylic Suspension
In early gnathostomes (e.g., placoderms), the mandibular arch (and especially the palatoquadrate) was anchored to the chondrocranium. This primitive autostylic suspension allowed the palatoquadrate to act as a lever, with opening and closing primarily happening via the dentary (Figure 8.19). The hyomandibula was not attached to either the chondrocranium or the palatoquadrate and did not aid in the opening of the jaws. This early style of jaw suspension allowed for jaw opening and closing, but little processing or “biting” could occur.
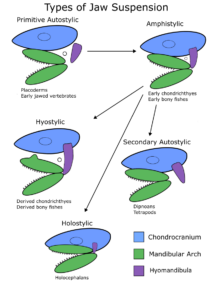
Amphistylic Suspension
From this primitive jaw suspension evolved amphistylic suspension, found in early chondrichthyans and primitive bony fishes. In this style of suspension, the palatoquadrate remains attached to the chondrocranium, while the hyomandibula is attached to the otic capsule of the chondrocranium and extends to attach to the posterior tip of the palatoquadrate (Figure 8.19). This produces multiple articulation points in the jaw, greater power, and therefore additional biting and chewing.
Hyostylic Suspension
From amphistylic suspension evolved hyostylic jaw suspension, found in derived chondrichthyans and bony fishes. The palatoquadrate is no longer attached to the chondrocranium, and instead the main articulation point is the hyomandibula (Figure 8.19). It remains attached to the otic capsule and the palatoquadrate. While (often) reducing the “power” generated by the jaws, this allows for jaw protrusion and rapid buccal expansion, which generates the negative pressure required for suction feeding. The jaws are able to move forward and downward, and sometimes both, during this movement. In some modern sharks, there is a process on the top of the palatoquadrate, preventing side-to-side motion of the jaws and limiting motion anteriorly and ventrally.
Secondary Autostylic Suspension
Secondary autostylic suspension evolved twice (independently and in different ways) from hyostylic jaw suspension. In holocephalans (e.g., chimeras), the palatoquadrate reattached itself to the chondrocranium, while the hyomandibula remained attached to both the chondrocranium and the palatoquadrate (Figure 8.19). This leads to a restricted version of the primitive autostylic jaw suspension (sometimes called holostylic jaw suspension), with simple jaw opening and closing and little jaw protrusion.
In Dipnoi and tetrapods, autostylic jaw suspension reevolved, leading to a reattached palatoquadrate and a hyomandibula that is only attached to the otic capsule (Figure 8.19). In this type of jaw suspension, that increased “power” in jaw motion reemerged and the ability to protrude the jaws decreased. In these lineages, toothplates often appeared, aiding in the biting, chewing, and processing of hard prey (durophage).
Jaw Articulation and Middle Ear Bones
Fishes
As discussed in the previous sections, the jaws of the majority of vertebrates (the fishes) include the “upper jaws” (the premaxilla and maxilla) and the “lower jaws” (the mandible consisting of the dentary and the angular-articular fused together). In this group, jaw articulation refers to the connection between the quadrate and angular-articular (Figure 8.20). This articulation point allows for the opening and closing of the jaw by rotating the mandible around this joint. This rotation and the resulting movement of the lower jaws allows for biting, rapid expansion of the mouth, and (primarily) aquatic feeding. Additional work has found another joint in some teleost feeding apparatuses: the teleost intramandibular joint (TIJ). This secondary joint allows for additional rotation of the bones associated with feeding, allowing for mouth closing while the jaws remain protruded. This possibly allows for additional methods of prey capture. See Section 8.7 on feeding modes for more details about fish jaws.

Figure 8.20—Jaw articulation in vertebrates: The connection between the quadrate and the angular-articular allows for the opening and closing of the jaws, but this is different across vertebrates (especially tetrapods).
Amphibians and Reptiles
To set the stage for changes that occur in mammals, it is helpful to remind ourselves of what the common ancestor may have looked like, using the amphibians and reptiles as a useful reference point. The mandible in amphibians and reptiles is made up of several bones, with only the dentary having teeth (Table 8.1). The upper jaw will also contain several bones, including the maxilla, premaxilla, quadrate, and quadratojugal. The jaw articulation joint in our amphibians and reptiles is the same as it was in our fishes, the quadrate and angular-articular bones (Figure 8.20). However, there is one element that we must discuss, as it will influence how we consider the changes that are going to occur in mammals. The bone is known as the columella, and at first glance, its very appearance may cause considerable confusion. The columella is a long, thin bone that originates on the braincase. In our frogs, the columella runs from the braincase to the tympanum, the large circular structure visible in frogs like the American bullfrog. For most reptiles, the tympanum is not so obvious from the surface, instead found deep to an external ear opening. As you have probably surmised, the columella plays a role in sound reception and transduction. However, many reptiles also pick up low-frequency vibrations from the ground through their jaws when they are resting.
Double Articulation in Mammals!
As discussed earlier (see Box 8.1—Temporal Roof Evolution), significant morphological changes happened in the jaws during this evolutionary period, leading to an alternative jaw joint and new auditory ossicles (bones that play a role in hearing or the transduction of sound). The exact evolutionary causes of these changes are unclear, but there are multiple factors that together clarify how these changes occurred and what allowed them to do so. The first, seemingly unrelated change to occur has to do with posture. In the evolution of mammals, they changed from a sprawling posture to more of an upright posture, with limbs tucked underneath the body (see Chapter 10—Appendicular Skeleton). This had the effect of raising the body and the head off the ground for longer periods of time. As we discussed in the last section, reptiles and amphibians could transmit sound through their lower jaw to the middle ear. With the head being raised off the ground for longer periods of time, that ability is diminished. But how do you create new ear bones and a new jaw joint without disrupting all the functions?
Thankfully, unlike many other systems, there is a robust fossil record that tells us exactly how this occurred. In the synapsids, well before the rise of the true mammals, there are several fossils with very large dentary bones, so large that they almost make contact with the squamosal bone, part of the braincase (Figure 8.20). Eventually, the continued increase in the size of the dentary directly results in it making contact with the squamosal bone. This led to the evolution of two distinct articulations of the lower jaw with the skull, and we can see this in stem mammals like Morganucodon (Figure 8.21; PBS—Your Inner Fish). These two joints sit right next to each other, with the dentary-squamosal joint being just lateral to the quadrate-articular joint. At the same time, the increasing braincase size and presence of the dentary-squamosal joint push the quadrate into smaller and smaller spaces, drastically reducing its size from what we saw in the reptiles. The new joint between the dentary and squamosal became the primary joint for chewing. This took pressure off the smaller quadrate and articular during feeding. This led to the evolution of new adaptations in these bones. Specifically with this new dentary-squamosal joint, the quadrate and articular bones are free to change. Their feeding function is now redundant; their primary function became hearing. There are many transitional forms, with some stem mammals having middle ear bones that functionally only provide support for hearing but are still firmly anchored to the dentary (now mandible). Over time, the middle ear bones become fully independent of the mandible (Figure 8.21). What once was the columella is now the stapes, the quadrate is now the incus, and the articular is now the malleus. The three bones shift in size and position, growing ever smaller and shifting away from the jaws. By the time we reach the extant mammals, they are completely converted to their role in hearing, and only developmentally can you see their history as the original jaw joint.

Figure 8.21—The evolution of the jaw joint and middle ear bones in mammals.
Secondary and Tertiary Jaws
Pharyngeal Jaws
While the oral jaws (what we’ve previously discussed) were an incredible adaptation that allowed for better and more efficient prey capture, many species of teleosts took the idea of jaws one step further by evolving a secondary set of jaws, the pharyngeal jaws. These jaws typically sit in the throat and are modified gill arches. Originally they were thought to only have evolved once, but newer research estimates at least two evolutions of pharyngeal jaws, with others hypothesizing closer to six instances (Wainwright et al., 2012).
The pharyngeal jaws are considered one of the “key innovations” among vertebrates, drastically improving prey capture and processing and allowing for the rapid diversification of teleosts. The existence of this secondary set of jaws means the oral jaws no longer need dual purposes (both capturing and processing prey prior to digestion) but instead can be the primary mode of prey capture. Prey processing is then accomplished by the pharyngeal jaws, further inside the organism (What’s Inside a Moray Eel’s Mouth? | Natural History Museum). This duality was thought to have allowed for the independent evolution of each system of jaws, increasing diversification of prey items and speciation. Recent work has put this idea into question: The two systems of jaws appear to be heavily connected and evolving together rather than independently. This likely means there are intense evolutionary pressures for these jaw systems to remain (somewhat) the same throughout evolutionary history in actinopterygians.
Tongue-Bite-Apparatus
One group of teleosts, Osteoglossomorpha, independently evolved a second set of jaws within the oral cavity, dubbed the tongue-bite apparatus (TBA). This jaw system consists of patches of teeth on the parasphenoid (on the roof of the mouth) and on the enlarged hyoid and branchial arches. The TBA is incredibly diverse in its size and dentition but, like the pharyngeal jaws, allows for food processing while leaving the oral jaws available for prey capture. Some species have reevolved pharyngeal jaws in addition to retaining the TBA, leaving them with three sets of jaws.
8.6 Teeth
The changes discussed in the previous section (Section 8.5, The Jaws) lead directly to changes in dentition and teeth in terms of location, type, and number of teeth. Cyclostomes (hagfishes and lampreys) have keratinous cones on the mouth and tongue, while the evolution of jaws brought about “true teeth.” It’s also worth noting that the development of teeth (and the materials they are made of) is a similar process to that of scales. In fact, it is likely that teeth evolved from scales when jaws began to form.
Structure
In general, a tooth has several layers surrounding the pulp cavity, where the blood vessels and nerves are maintained. These layers include hard enamel covering a bone-like substance called dentine (together forming the “crown” above the gum line). Below the gum line, the pulp cavity is further protected by cement, the periodontal ligaments, and the jawbone itself.
Development
Tooth development originates from two cell sources: epithelium and mesenchyme cells. The epithelium is derived from oral ectoderm, while the mesenchyme is derived from cranial neural crest cells (for more information about these cell types, see Chapter 6—Integument). Initiation of tooth development when specific genes are expressed in sites where the teeth will form lead to the thickening of the oral epithelium. We’ll use Figure 8.22 to show the following process of tooth development.

Figure 8.22—The development of teeth from early embryonic stages, showing the morphological progression and the physiological stages. Based on Thesleff, 2014.
The bud stage immediately afterward is when that epithelial thickening increases and forms the dental lamina, pushing into the dental mesenchyme. This push of the dental lamina creates the epithelial bud, which in turn forces the mesenchyme cells directly below them to condense. Those mesenchyme cells form the dental papilla. The epithelial bud continues to grow, surrounding the dental papilla. The mesenchyme cells in and around the dental papilla form the dental follicle or sac. The sac starts to increase in size, and the crown begins to develop (producing the tooth!). The shape of the tooth is determined at this stage: whether there will be one or two enamel knots (i.e., the early stages of cusps) resulting in single-cusped teeth or molars, respectively.
Tooth-specific cell types (odontoblasts and ameloblasts) begin to differentiate at this time. The ameloblasts are responsible for producing enamel (the incredibly hard coating around the teeth). The odontoblasts produce dentin, found in the center of the dental papilla. When the tooth is formed (the crown is present), the roots are formed and the tooth erupts.
Types of Teeth
Some organisms have variation in tooth size and shape, while others have incredibly similar tooth features across the mouth. In bony fishes, amphibians, and reptiles, shape tends to remain superficially consistent in the mouth (morphologically homodont), although size can vary. Common tooth shapes in these groups include conical, triangular/bladelike (i.e., great white sharks), peg-like (molariform), or platelike formations of teeth called toothplates (Figure 8.23).

Figure 8.23—Representative tooth shapes in fishes, amphibians, and reptiles. (A) Triangular tooth of the great white shark, Carcharodon carcarius; (B) Conical teeth of the Komodo dragon, Varanus komodoensis; (C) Peg-like teeth of the Savannah monitor; Varanus exanthematicus; (D) Toothplate of cownose ray, Rhinoptera bonasus.
In mammals, however, tooth size and shape tend to change across the jaws (morphologically heterodont). We can likely picture this by looking inside our own jaws. Placental mammals have matching teeth on either side of the jaws. In primitive mammals, each side (typically) included three incisors found on the rostral part of the jaws, followed by one canine, four premolars, and three molars (Figure 8.24).

Figure 8.24—Mammalian tooth types as represented by a human.
This pattern is still found in some mammals, although most mammals (including humans) have lost some of these teeth, altering their pattern. Types of teeth can be documented using a dental formula, which describes the teeth found on the upper jaw (numerator) and the lower jaw (denominator) on a given side of the organism. In this formula, incisors are abbreviated “I,” canines with “C,” premolars with “P,” and molars with “M.” The common mammal dental formula would be I 3/3, C 1/1, P 4/4, M 3/3. In humans, this shifts to I 2/2, C 1/1, P 2/2, M 3/3.
We would be remiss to ignore some of the odd teeth found in the vertebrate lineage. Enlarged and elongated incisors are often used by mammals for mating purposes, both for physical altercations and for secondary sexual characteristics. Tusks in elephants and Artiodactyla (e.g., wild boar, hippopotamuses) are large teeth, protruding from the skull for combat. Narwhals, also in the Artiodactyla clade, have a singular enlarged incisor, giving them a unique appearance (Figure 8.25).

Figure 8.25—Some examples of modified teeth (tusks) in mammals. (A) Narwhal; (B) Saber-tooth tiger; (C) Musk deer; (D) Elephant; (E) Warthog.
Substructures
Mammalian teeth often have multiple substructures that allow for multiple functions but also the closing of the jaws during biting (occlusion). You can picture a set of alternating triangles fitting together nicely but often with slightly more complex shapes. We often describe these substructures by the side (toward the tongue or the cheek) of the tooth and by the shape. Structures on the tongue side of the tooth are called lingual, while those on the cheek side are labial. Over time, cones and cusps evolved to increase the number of shearing surfaces on these teeth. In Carnivora, the third upper premolar and the first lower molar are called carnassials and are distinguished by their incredibly large shearing surface (Figures 8.26 and 8.27).

Figure 8.26—The carnassial teeth of several representative mammals. (A) European brown bear, Ursus arctos; (B) Leopard, Panthera pardus; (C) Domestic dog, Canis lupus familiaris; (D) European badger, Meles meles.

Figure 8.27—Different tooth shapes and styles in mammals.
In herbivores and omnivores, crushing surfaces are emphasized over shearing surfaces. Bunodont molars have rounded cusps for crushing and grinding and are typically seen in humans, pigs, and more basal herbivores (Figure 8.27). In contrast, sometimes those cusps fuse together to form ridges in lophodont molars in more specialized herbivores (Figure 8.27). Taking it a step further, those ridges become even taller and longer, eventually forming crescents in selenodont molars found in Artiodactyls (Figure 8.27).
Bunodont molars tend to remain fairly low-crowned (brachydont), with their height not extending significantly past the gums (Figure 8.28). Lophodont and selenodont molars are typically high-crowned (hypsodont, with their ridges and crescents extending far beyond the line of the gums; Figure 8.27).

Figure 8.28—Hypsodont and brachydont teeth.
Tooth Attachments
There are several different types of tooth attachment, primarily distinguished by the degree to which the teeth are “set” in the jawbone. Superficial attachment can be acrodont or pleurodont. Acrodont teeth are attached to the top or inside edge of the jaw, and there are limited options for replacement (Figure 8.29A). Pleurodont teeth are attached to the outside edge of the jaw (Figure 8.29B). Thecodont teeth are set in deep sockets in the jawbone itself and evolved in the ancestors to mammals. They can have one or more roots and are able to withstand greater pressures, and this allows for greater diet disparity (Figures 8.29C and 8.29D).

Figure 8.29—Different styles of tooth attachment to the jaws.
Replacement
The majority of vertebrates (i.e., most fishes) are polyphyodont; new teeth can develop as old teeth wear and/or fall out during an organism’s life. The new tooth begins to develop prior to the old tooth being lost, leaving little (or no) time when the organism is missing necessary teeth. This is a very complex (and consistent) process. In bony fishes, activation waves of replacement happen from the anterior to posterior part of the jaw. When the new tooth matures, the root is reabsorbed, allowing the old tooth to loosen and fall out. This often leads to the jaw containing a combination of teeth: brand-new teeth that just came in, empty sockets with new teeth erupting soon, and fully formed, mature teeth. The empty sockets do not typically stay empty for long; the activation waves ensure that the majority of teeth are either new or fully mature at any given moment.
Not all vertebrates replace teeth like this; the majority of mammals are diphyodont (Greek “diphy” = two), including humans. These organisms have two sets of teeth: milk (deciduous) teeth and permanent teeth. The permanent teeth replace the milk teeth during the prolonged maturation time. Toothed whales (e.g., dolphins, porpoises, sperm whales) are monophyodont, only possessing one set of teeth.
For some organisms, diet plays a crucial role in tooth wear and, therefore, replacement. In most mammals, including humans, incisors are relatively small and typically used for picking up food or making small cuts. In rodents, incisors have been reduced in number but increased in terms of size. Their two pairs of incisors (instead of the usual four) constantly grow because they do not seal their pulp cavity. Rodents typically consume plant materials that wear down teeth quickly. Unless addressed, this problem would result in rodents replacing their incisors constantly. Instead, the wear from eating plants keeps the incisors from overgrowing while removing the need for full tooth replacement. The most anterior part of the tooth is made of enamel, the harder material, which isn’t worn down as quickly. The posterior part of the tooth is dentine, which is worn down but also replaced at a faster rate. The resulting teeth have a hard, sharp frontal edge, which is great for cutting.
Function
Different tooth types tend to relate directly to diet. For instance, conical, pointy teeth are much more efficient at piercing prey to prevent its escape, while serrated teeth (like those in some sharks) are better at cutting prey into digestible pieces. Flat-topped, molariform teeth and toothplates are used for crushing hard prey items while reducing stress on the jaws.
Heterodonty Versus Homodonty
An important consideration when thinking about dentition is the relative size, shape, and position of the teeth within the jaw itself. Position in the tooth row has a direct connection to the amount of pressure or stress a given tooth will be under during a biting motion. If we consider an idealized dentary (Figure 8.30) and place idealized conical teeth on it, we can imagine different stress loads based on the size of those teeth. For instance, in Figure 8.30, all the teeth are exactly the same size from the front of the dentary to the back, making them morphologically homodont. When considering the stress changes across the dentary, however, it is easy to picture the teeth in the back of the jaw experiencing additional pressure or stress during the jaw closing, as they quickly approach the upper palatoquadrate. This means that this jaw example is both morphologically homodont and functionally heterodont. The teeth across the jaw are experiencing different stresses. Alternatively, in the bottom right illustration of Figure 8.30, the size of the teeth changes across the jaw, while the pressure experienced by each tooth remains fairly consistent. This jaw example would be considered morphologically heterodont but functionally homodont.

Figure 8.30—Morphological versus function heterodonty and homodonty. Based on Cohen et al., 2020.
8.7 Feeding Modes
Ram Feeding
With the evolution of jaws and variable dentition came the need to use those jaws for prey capture in aquatic environments (see also Chapter 5). The earliest form of feeding with jaws came from simply opening the mouth using head (epaxial) and body (hypaxial) muscles. The epaxial muscles lifted the top of the head, while the hypaxial muscles and interhyoideus pulled the dentary down and backward. This motion wasn’t incredibly fast but allowed the mouth to open wide. Importantly, the overall volume of the mouth did not increase. The organism (e.g., whale shark) could swim with its mouth open or could open it immediately prior to swimming upon the prey. Many larger aquatic vertebrates use this method of feeding and eat many small prey items during a feeding event. They often have extensive gill rakers to assist with sorting the prey from the water, as water was brought into the mouth at the same time as the prey.
Suction Feeding
We saw a new method of feeding evolve in chondrichthyans and early actinopterygians, but it was improved dramatically in Teleostei. During the evolution of the Teleostei from the more basal actinopterygians, we saw a drastic increase in the number of bones in the dermatocranium, and these bones became incredibly mobile. These changes allowed for significant changes in the overall mobility of the jaw and for complex movements to happen (nearly) simultaneously. In suction feeding, several things happen in very quick succession: The epaxials lift the head up, the hypaxials pull the dentary ventro-caudally, and the levator operculi rotates the gill coverings, further rotating the dentary, premaxilla, and maxilla (Figure 8.31). The rotation of the premaxilla and maxilla causes the upper jaws to protrude (they are practically thrown forward), quickly expanding the volume of the mouth cavity and generating suction (Fish Suction Power: Dynamic Digital Endocast). This suction rapidly brings both water and prey into the mouth for processing (Epibulus insidiator, the slingjaw wrasse). While this increased range of motion is incredibly beneficial to adult organisms, it can lead to challenges in larval and juvenile fishes who aren’t able to produce enough suction to adequately feed. Many larval fishes aren’t able to survive due to a lack of suction power (Larval fish failing to suction feed at 1,000 frames per second).

Figure 8.31—The movements of the skull bones during suction feeding in a largemouth bass, Micropterus salmoides.
Biting/Chewing
Many organisms, including some fishes, rely on biting and/or chewing when processing prey, and this is often visualized by dentition type and pattern in both the oral jaws and the pharyngeal jaws (e.g., conical teeth for piercing versus molariform for crushing, as discussed in Section 8.6, Teeth). Different tooth types likely influence what prey items are processed by an organism, but other changes in the skull were also necessary to allow for true “biting” to occur. Bones tend to be more robust or well-ossified if the organism bites or chews its food, usually brought about by the need for strong muscles and muscle attachments on the head. Each “bite” is produced by torque around the jaw joint, generating force via the teeth onto the prey item. This biting action is true for both terrestrial and aquatic organisms, but they are forced to go about these adaptations in slightly different ways due to the mediums in which they live.
8.8 The Water-to-Land Transition—Tongues and Jaws
One of the key elements in the history of vertebrates was the transition from water to land. This transition impacted so many of the systems in this book, and the jaws certainly exemplify this transition. We will see that not only do the jaws change during the transition, but the lasting impact of that transition will result in continual changes for a considerable length of time.
To understand some of the “why” before the “what,” let us revisit the previous section on how vertebrates feed using their jaws. In the aquatic environment, suction feeding is the dominant mode, along with suspension feeding (filter feeding). Both of these modes require food suspended in the environment. When a largemouth bass sucks in the small minnow, it is not just sucking in the minnow but also ingesting a large volume of water. In fact, you could look at the situation as being reversed: The bass sucks in a large volume of water, and the minnow is carried in with it. Once an animal leaves the aquatic environment, it no longer has the luxury of being able to move the environment and capture the food contained within that environment. Instead, the animal in question must be able to only manipulate the food item itself. This predominantly limits the system to the biting/chewing system described above. In response to this style, you can see the changes to the skull in our early tetrapods: significant fusions of the skull bones and a complete loss of kinesis. This is the result of all the additional forces on the skull.
The second aspect of this transition is what happens once the food is in the mouth. In the water, as long as you can move the water, you can move your food. Transport of food in the mouth and into the esophagus requires moving the water. However, in the terrestrial environment, the anatomical structures are now required to both support and move the food in the mouth and into the esophagus. As we discussed in the section introducing tetrapods (and the gross muscle anatomy in Chapter 12), the skeletal and muscular elements that made up the gills are modified to become the tongue and its skeleton. The highly mobile tongue and its supporting elements play a large role in the ability to move food within the mouth and into the esophagus. Independently, in each group of tetrapods, the tongue has evolved to act not only as food transport but also directly for food capture. Frogs, salamanders, chameleons, woodpeckers, and anteaters all use their tongues to capture their prey.
8.9 Summary
The vertebrate skull is an extremely dynamic structure that has changed greatly through evolutionary time. As the center for the senses, brain, and feeding, it has been shaped and fine-tuned by natural selection across millions of years. The differences between individual species (and even individuals within a species) are extremely insightful into a species’ history, evolution, and ecology.
Application Questions
- The dinosaurs, despite being diapsids, have more than two fenestrae found throughout their skull. What is the likely functional reason for this change?
- Why is the trade-off between force and mobility so apparent in skulls?
8.10 Further Reading
- Dickinson, Edwin, Melody W. Young, and Michael C. Granatosky. “Beakiation: How a novel parrot gait expands the locomotor repertoire of living birds.” Royal Society Open Science 11, no. 1 (2024): 231397.
- Fabbri, Matteo, Guillermo Navalón, Nicolás Mongiardino Koch, Michael Hanson, Holger Petermann, and Bhart-Anjan Bhullar. “A shift in ontogenetic timing produced the unique sauropod skull.” Evolution 75, no. 4 (2021): 819–831.
- Hanken, J., and B. K. Hall. “The Skull, Volume 1–3.” Development. The University of Chicago Press: Chicago (1993).
- Holliday, Casey M., William Ruger Porter, Kent A. Vliet, and Lawrence M. Witmer. “The frontoparietal fossa and dorsotemporal fenestra of archosaurs and their significance for interpretations of vascular and muscular anatomy in dinosaurs.” The Anatomical Record 303, no. 4 (2020): 1060–1074.
- Jung, Jae-Young, Andrei Pissarenko, Adwait A. Trikanad, David Restrepo, Frances Y. Su, Andrew Marquez, Damian Gonzalez, Steven E. Naleway, Pablo Zavattieri, and Joanna McKittrick. “A natural stress deflector on the head? Mechanical and functional evaluation of the woodpecker skull bones.” Advanced Theory and Simulations 2, no. 4 (2019): 1800152.
- Nweeia, M. T., Nutarak, C., Eichmiller, F. C., Eidelman, N., Giuseppetti, A. A., Quinn, J., Mead, J. G., K’issuk, K., Hauschka, P. V., Tyler, E. M. and Potter, C. W., 2009. “Considerations of anatomy, morphology, evolution, and function for narwhal dentition.” Smithsonian at the Poles: Contributions to International Polar Year Science.
8.11 References
- Anthwal, Neal, Jane C. Fenelon, Stephen D. Johnston, Marilyn B. Renfree, and Abigail S. Tucker. “Transient role of the middle ear as a lower jaw support across mammals.” eLife 9 (2020): e57860.
- Camp, Ariel L., Thomas J. Roberts, and Elizabeth L. Brainerd. “Swimming muscles power suction feeding in largemouth bass.” Proceedings of the National Academy of Sciences U.S.A. 112, no. 28 (2015): 8690–8695.
- Cohen, Karly E., Hannah I. Weller, and Adam P. Summers. “Not your father’s homodonty—stress, tooth shape, and the functional homodont.” Journal of Anatomy 237, no. 5 (2020): 837–848.
- Felice, Ryan N., Akinobu Watanabe, Andrew R. Cuff, Michael Hanson, Bhart-Anjan S. Bhullar, Emily R. Rayfield, Lawrence M. Witmer, Mark A. Norell, and Anjali Goswami. “Decelerated dinosaur skull evolution with the origin of birds.” PLoS Biology 18, no. 8 (2020): e3000801.
- Galatius, Anders, Rachel Racicot, Michael McGowen, and Morten Tange Olsen. “Evolution and diversification of delphinid skull shapes.” Iscience 23, no. 10 (2020).
- Gibb, Alice C., Katie Staab, Clinton Moran, and Lara A. Ferry. “The teleost intramandibular joint: A mechanism that allows fish to obtain prey unavailable to suction feeders.” Integrative and Comparative Biology 55, no. 1 (2015): 85–96.
- Jarvik, Erik. Basic Structure and Evolution of Vertebrates. United Kingdom: Academic Press, 1980.
- Jheon, Andrew H., Kerstin Seidel, Brian Biehs, and Ophir D. Klein. “From molecules to mastication: The development and evolution of teeth.” Wiley Interdisciplinary Reviews: Developmental Biology 2, no. 2 (2013): 165–182.
- Kuratani, Shigeru. “Craniofacial development and the evolution of the vertebrates: The old problems on a new background.” Zoological Science 22, no. 1 (2005): 1–19.
- Rhoda, Daniel, P. David Polly, Christopher Raxworthy, and Marion Segall. “Morphological integration and modularity in the hyperkinetic feeding system of aquatic-foraging snakes.” Evolution 75, no. 1 (2021): 56–72.
- Thesleff, I. “Current understanding of the process of tooth formation: Transfer from the laboratory to the clinic.” Australian Dental Journal 59 (2014): 48–54.
- Thiruppathy, Mathi, Peter Fabian, J. Andrew Gillis, and J. Gage Crump. “Gill developmental program in the teleost mandibular arch.” eLife 11 (2022): e78170.
- Wainwright, Peter C., Smith, W. Leo, Price, Samantha A., Tang, Kevin L., Sparks, John S., Ferry, Lara A., Kuhn, Kristen L., Eytan, Ron I., and Near, Thomas J. “The evolution of pharyngognathy: A phylogenetic and functional appraisal of the pharyngeal jaw key innovation in labroid fishes and beyond.” Syst. Biol. 61, no. 6 (2012): 1001–1027.
Media Attributions
- Figure 8-12 – Temporal Roof v2
- Figure 8.19 © Kassandra Ford and Bill Ryerson is licensed under a CC BY (Attribution) license

